Phenylamine & Azo Compounds
This lesson covers:
- How phenylamine is prepared from nitrobenzene
- The reaction of phenylamine with aqueous bromine
- How phenylamine is used to synthesise azo dyes
- How phenol is prepared from phenylamine
Reducing nitrobenzene to phenylamine
Phenylamine (C6H5NH2) can be prepared by reducing nitrobenzene (C6H5NO2) with tin and concentrated hydrochloric acid.
The reduction involves:
- Heating nitrobenzene under reflux with tin and concentrated HCl. This provides the reducing conditions.
- The organic product formed is the ammonium salt, phenylammonium chloride (C6H5NH3+Cl-).
- The ammonium salt is then neutralised by adding aqueous sodium hydroxide to give phenylamine (C6H5NH2).
The overall reaction can be summarised as:
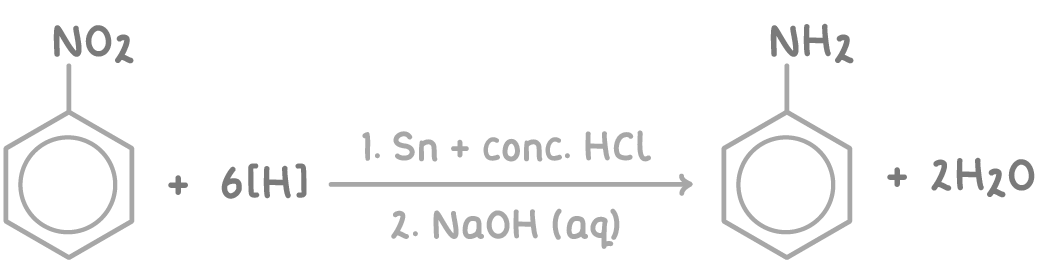
The phenylamine product is separated from the reaction mixture by steam distillation.
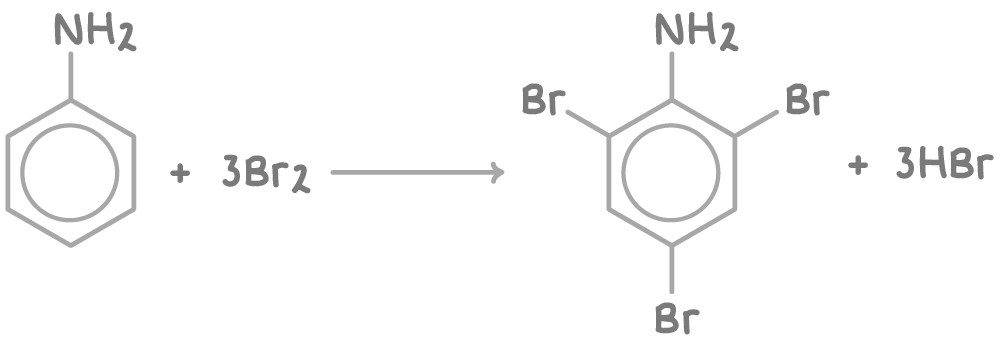
Phenylamine with aqueous bromine
When phenylamine reacts with aqueous bromine at room temperature, electrophilic substitution occurs at the 2, 4, and 6 positions of the benzene ring.
This is because the -NH2 group is electron donating, activating these positions towards electrophilic attack.
The reaction produces 2,4,6-tribromophenylamine as a white precipitate:
Using phenylamine to make azo dyes
Phenylamine is an important starting material for producing brightly coloured dyes called azo dyes.
Azo dye synthesis involves two steps:
- Diazotisation of phenylamine into a benzenediazonium ion
- Coupling reaction with a phenolate ion to form the dye
Step 1 - Diazotisation
Initially, nitrous acid (HNO2) is produced in situ by reacting sodium nitrite with dilute HCl at temperatures below 10°C:
NaNO2(s) + HCl(aq) ➔ HNO2(aq) + NaCl(aq)
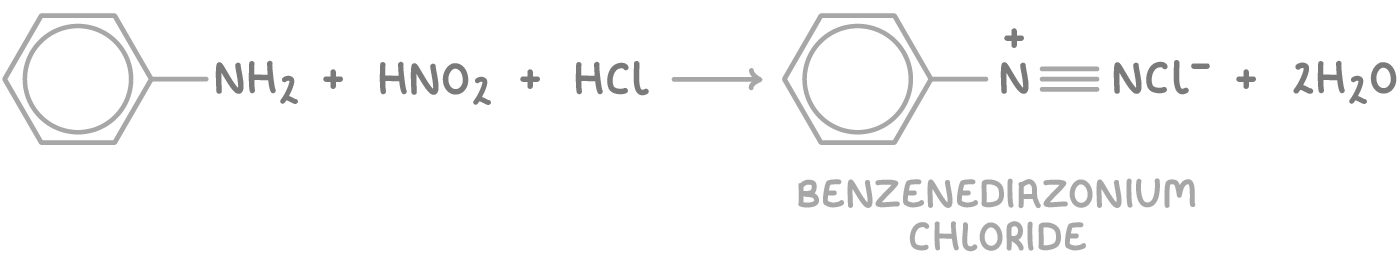
Subsequently, nitrous acid reacts with phenylamine in an electrophilic substitution to form a diazonium salt, specifically benzenediazonium chloride:
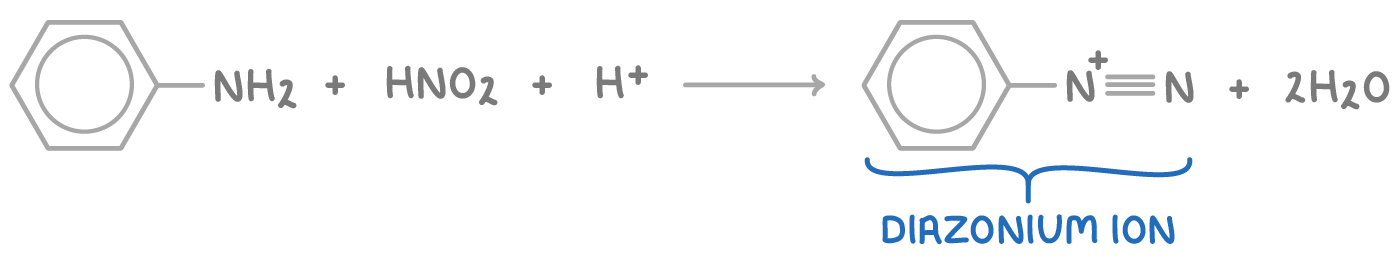
Nitrous acid is generated in situ because it is unstable, and this method allows for better control over the reaction rate and temperature, which must be kept below 10°C to prevent the decomposition of the unstable diazonium ion.
Step 2 - Coupling reaction
Next, the diazonium ion undergoes electrophilic substitution with phenol under alkaline conditions.
This produces an orange azo dye:
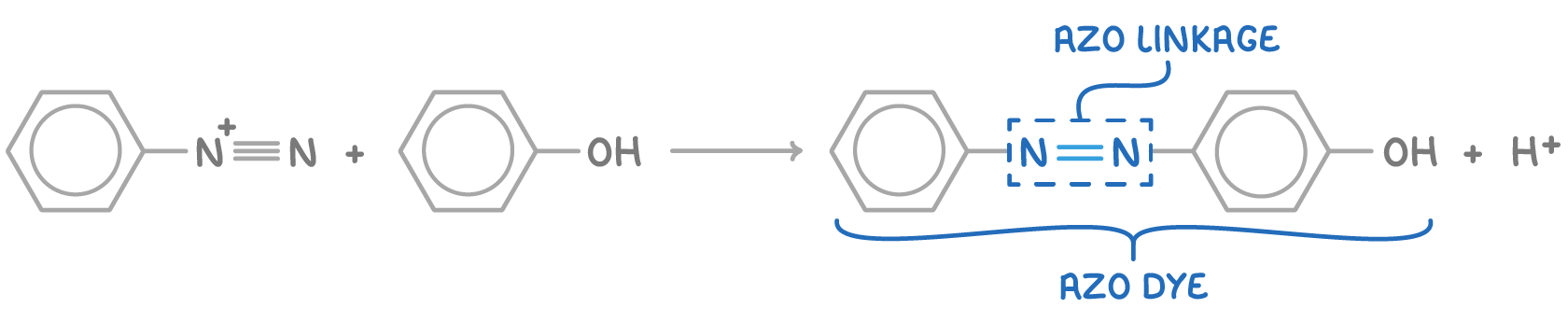
The distinctive feature of azo dyes is the azo linkage (N=N) that connects two aromatic rings. These dyes are characterised by their brightly coloured appearance and stability, which are a result of the extended delocalised π-system spanning the two aromatic rings and the azo linkage. Additionally, by using different aryl compounds in place of phenol, dyes of various colours can be synthesised.
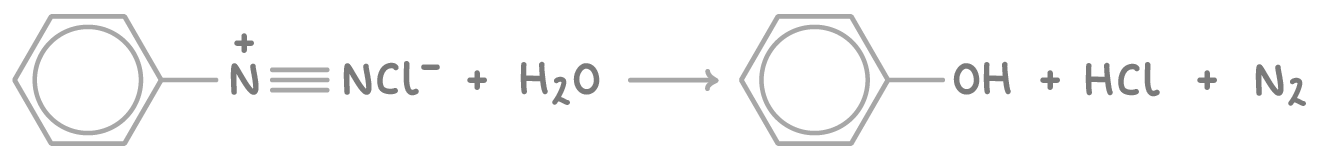
Preparing phenol via diazotisation
The diazotisation reaction can also be used to synthesise phenol from phenylamine.
This process involves a two-step reaction sequence, with the first step being the same as in azo dye synthesis.
- Diazotisation of phenylamine into a benzenediazonium ion
- Decomposition to form phenol
Step 1 - Diazotisation
Initially, nitrous acid (HNO2) is produced in situ by reacting sodium nitrite with dilute HCl at temperatures below 10°C:
NaNO2(s) + HCl(aq) ➔ HNO2(aq) + NaCl(aq)
Subsequently, nitrous acid reacts with phenylamine in an electrophilic substitution to form a diazonium salt, specifically benzenediazonium chloride:
Step 2 - Decomposition
In contrast to azo dye synthesis, where the diazonium salt undergoes a coupling reaction with a phenolate ion, the unstable diazonium salt in this process is gently heated in water, causing it to decompose and form phenol: