Oxidation of the Alkenes
This lesson covers:
- Oxidation of alkenes with cold, dilute acidified potassium manganate(VII)
- Oxidation of alkenes with hot, concentrated acidified potassium manganate(VII)
- How oxidation products indicate alkene structure
Cold, dilute oxidation forms diols
When alkenes react with cold, dilute acidified potassium manganate(VII) (KMnO4(aq)), they undergo a chemical change known as oxidation.
- The pale purple KMnO4 solution changes to colourless as the permanganate ions (MnO4-) are reduced to manganese(II) ions (Mn2+).
- In this reaction, each carbon atom in the C=C double bond gains an -OH group. This forms a compound called a diol, which has two alcohol groups.
For instance, ethene reacts with KMnO4(aq) to produce ethane-1,2-diol:
H2C=CH2 + [O] + H2O ➔ HO-CH2CH2-OH
This reaction is significant because it can be used to test for the presence of a C=C double bond in a compound, which is a characteristic feature of alkenes.
Hot, concentrated oxidation breaks C=C bonds
Using hot, concentrated acidified KMnO4(aq) results in a more intense oxidation of alkenes, leading to the complete breaking of the C=C double bond:
- The diol intermediate loses its -OH groups and gets further oxidised into ketones, aldehydes or carboxylic acids.
- The nature of the final oxidation products depends on the types of groups attached to the carbons of the C=C bond.
Oxidation products with hot concentrated KMnO4
The oxidation products formed when an alkene reacts with hot, concentrated acidified KMnO4 depend on the groups bonded to each carbon atom in the C=C double bond:
- Two hydrogens (=CH2) - If a carbon atom in the double bond is bonded to two hydrogen atoms, it will oxidise to carbon dioxide (CO2).
- One hydrogen + one alkyl group (=CHR) - If a carbon atom has one hydrogen atom and one alkyl group (R) bonded to it, it will oxidise to form a carboxylic acid (RCOOH).
- Two different alkyl groups (=CR1R2) - If a carbon atom has two different alkyl groups (R1 and R2) bonded to it, it will oxidise to form a ketone (R1R2C=O).
| Alkene | Oxidation product | Example reaction |
|---|---|---|
| H2C=CH2 | Carbon dioxide (CO2) | H2C=CH2 + 6[O] ➔ 2CO2 + 2H2O |
| RHC=CHR | Carboxylic acid (RCOOH) | RHC=CHR + 4[O] ➔ 2RCOOH |
| R1R2C=CR1R2 | Ketone (R1R2C=O) | R1R2C=CR1R2 + 2[O] ➔ 2R1R2C=O |
When an unsymmetrical alkene containing two different groups on either side of the C=C double bond is oxidised, two different oxidation products will be formed, one from each side of the double bond.
For example, oxidising (CH3)2C=CH2 would give a ketone, (CH3)2C=O, from one side of the double bond and carbon dioxide (CO2) from the other side.
Using oxidation products to deduce alkene structure
The harsh oxidation conditions break and alter the original structure of the alkene.
However, by examining the oxidation products, we can infer structural information about the original alkene:
- Carbon dioxide - This indicates that the double bond was between two terminal carbons in the chain.
- Ketone - Suggests that each of the double bonded carbons was originally attached to two alkyl groups.
- Carboxylic acid - Implies that one of the double bonded carbons was initially bonded to an alkyl group, and the other to a hydrogen atom.
So the specific oxidation products provide vital clues about the identity of unknown alkenes.
Worked example 1 - Determining alkene structure from oxidation products
Given the oxidation products as carbon dioxide and propanoic acid, determine the possible structure of the original alkene.
Step 1: Interpretation of oxidation products
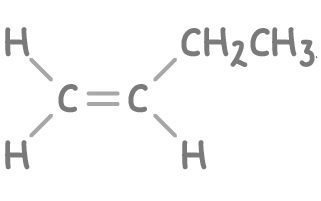
- Carbon dioxide - Indicates a carbon atom in the double bond was bonded to two hydrogen atoms.
- Propanoic acid (CH3CH2COOH) - Suggests the other carbon in the double bond was bonded to an ethyl group (CH3CH2) and a hydrogen atom.
Step 2: Constructing the alkene structure
- The alkene must have four carbon atoms.
- The carbon that produced carbon dioxide was a terminal carbon (at the end of the chain) with two hydrogens.
- The carbon that produced propanoic acid was a middle carbon, attached to an ethyl group and a hydrogen atom.
A possible structure of the original alkene could be but-1-ene:
Worked example 2 - Determining alkene structure from oxidation products
Given the oxidation products as propanone and butanone, determine the possible structure of the original alkene.
Step 1: Interpretation of oxidation products
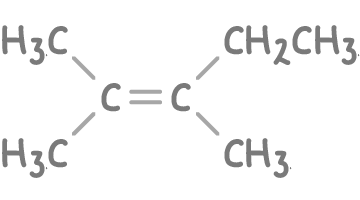
- Propanone (CH3COCH3) - Indicates one of the carbons in the double bond was bonded to two methyl groups (CH3).
- Butanone (CH3CH2COCH3) - Suggests the other carbon in the double bond was bonded to a methyl group (CH3) and an ethyl group (CH3CH2).
Step 2: Constructing the alkene structure
- The alkene must have seven carbon atoms.
- The carbon that produced propanone was a middle carbon, attached to two methyl groups
- The carbon that produced butanone was a middle carbon, attached to a methyl group and an ethyl group.
A possible structure of the original alkene could be 2,3-dimethylpent-2-ene: