Esters
This lesson covers:
- The structure and naming of esters
- Methods to make esters
- Hydrolysis of esters
Esters contain the functional group -COO-
Esters are organic compounds formed from the reaction between a carboxylic acid and an alcohol, where a molecule of water is removed during the process. The general structure of an ester is R-COO-R’.
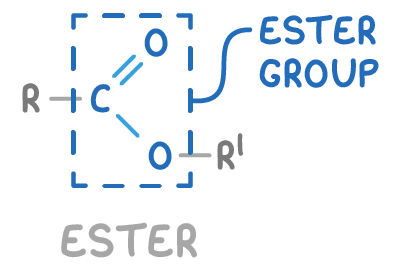
Where:
- R is an alkyl or aryl group from the carboxylic acid.
- COO- is the ester functional group.
- R’ is an alkyl or aryl group from the alcohol.
Naming esters
The name of an ester consists of two parts:
- The first part comes from the alcohol used and is the alkyl (R) group.
- The second part comes from the carboxylic acid used. The acid ending is replaced by 'oate' to give the second part of the name.
For example, propyl ethanoate is formed from propanol and ethanoic acid:
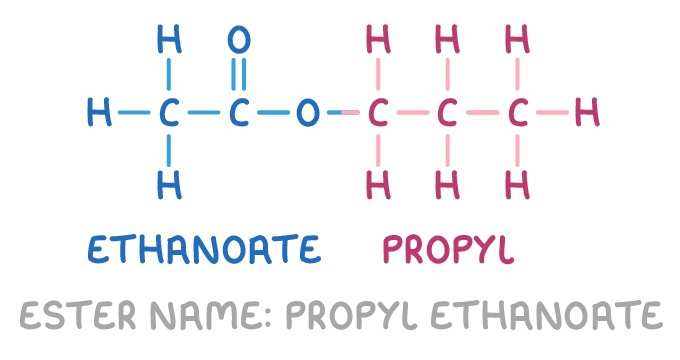
Synthesising esters
There are primarily two methods you need to know for synthesising esters.
1. From alcohols and carboxylic acids
- Mix the carboxylic acid and alcohol under reflux with an acid catalyst, such as H2SO4, in a reaction known as esterification.
- Applying an excess of alcohol can drive the reaction towards the formation of the ester product.
- The resulting ester can be isolated using fractional distillation.
- For example, the reaction to produce ethyl ethanoate from ethanol and ethanoic acid is:
C2H5OH + CH3COOH ⇌ CH3COOC2H5 + H2O
2. From alcohols and acyl chlorides
- Mixing acyl chloride with alcohol at room temperature leads to a rapid and vigorous reaction, producing an ester and hydrochloric acid gas.
- For example, the reaction to produce ethyl ethanoate from ethanol and ethanoyl chloride is:
C2H5OH + CH3COCl ➔ CH3COOC2H5 + HCl
Hydrolysis of esters
The process of hydrolysis breaks down esters into their original alcohol and carboxylic acid components using water.
There are two hydrolysis methods:
- Acid hydrolysis - The ester is heated under reflux with a dilute acid, such as HCl or H2SO4, to produce a carboxylic acid and an alcohol.
The general equation is: RCOOR’ + H2O ⇌ RCOOH + R’OH
- Base hydrolysis - When the ester is heated under reflux with a dilute base like NaOH, the products are a carboxylate salt and alcohol. This method is generally irreversible due to the stability of the carboxylate salt.
The general equation is: RCOOR’ + NaOH ➔ RCOONa + R’OH