Alcohols
This lesson covers:
- How alcohols react with acyl chlorides to form esters
- The nucleophilic addition-elimination mechanism
- The advantages of using acyl chlorides in ester synthesis
Alcohols react with acyl chlorides to form esters
In addition to reacting with carboxylic acids, alcohols can also react with acyl chlorides to form esters. Acyl chlorides contain a carbonyl group (C=O) bonded to a chlorine atom.
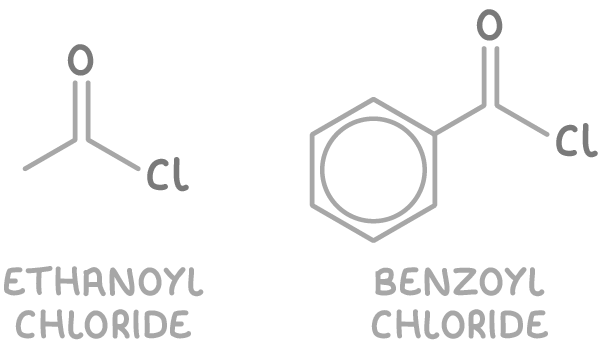
Examples of acyl chlorides include:
- Ethanoyl chloride (CH3COCl)
- Benzoyl chloride (C6H5COCl)
When an alcohol reacts with an acyl chloride, an ester is produced along with hydrogen chloride as a byproduct:
RCOCl + R'OH ➔ RCOOR' + HCl
For example, ethanol reacts vigorously with ethanoyl chloride to form ethyl ethanoate:
CH3COCl + C2H5OH ➔ CH3COOC2H5 + HCl
Acyl chlorides react via nucleophilic addition-elimination
The reaction between an alcohol and an acyl chloride occurs via a two-step nucleophilic addition-elimination mechanism:
- The oxygen's lone pair on the alcohol acts as a nucleophile, attacking the electrophilic carbonyl carbon of the acyl chloride.
- The C-Cl bond breaks as the R-O bond forms, displacing the chlorine as a chloride ion.
- The chloride ion removes a proton from the alcohol, forming hydrogen chloride (HCl).
For example, the mechanism using ethanoyl chloride and ethanol as the nucleophile is shown below:
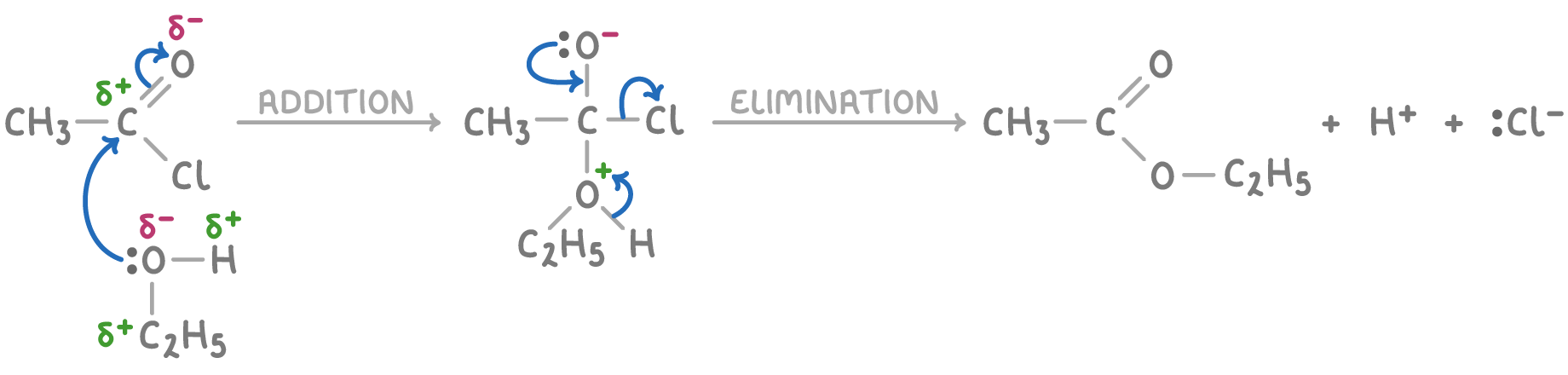
Acyl chlorides react faster than carboxylic acids
The reaction of acyl chlorides with alcohols/phenol occurs much faster than the analogous reaction with carboxylic acids.
There are two key reasons for the increased rate:
- The C-Cl bond is more polar than the O-H bond in carboxylic acids due to chlorine's greater electronegativity. This makes the acyl chloride more reactive.
- Unlike carboxylic acids, acyl chloride reactions with alcohols/phenol do not form an equilibrium mixture. Instead, the reaction goes to completion, driving the forward reaction.
Advantages of using acyl chlorides over carboxylic acids
The reaction of alcohols with acyl chlorides has two key advantages over reactions with carboxylic acids:
- Increased rate - Acyl chlorides react much faster than carboxylic acids. This is because the C-Cl bond in acyl chlorides is more polar than the O-H bond in carboxylic acids due to chlorine's greater electronegativity, making the acyl chloride more reactive.
- Higher yields - Acyl chloride reactions go to completion rather than reaching an equilibrium. This allows higher yields of esters to be obtained.
For example, reacting ethanoyl chloride with ethanol can yield nearly 100% of ethyl ethanoate, whereas the same reaction with ethanoic acid might only produce a 50% yield.