Synthetic Routes
This lesson covers:
- Summaries of key reactions for aliphatic compounds
- Summaries of key reactions for aromatic compounds
- How functional groups determine reactivity
Summary of key organic reactions for aliphatic compounds
Below is an overview of the primary types of organic reactions we've discussed for aliphatic compounds.
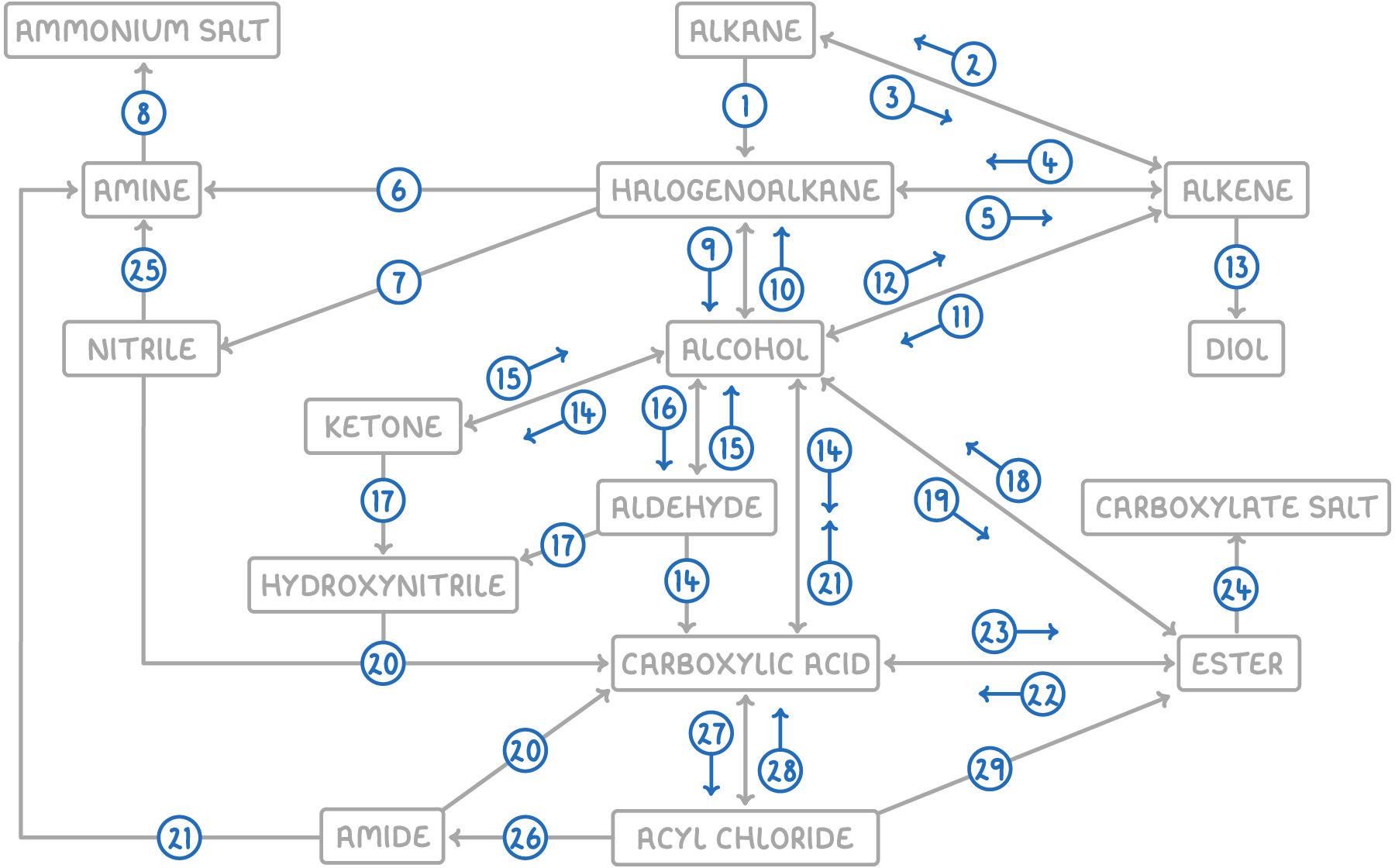
| Reaction | Reagent and conditions | Reaction type |
|---|---|---|
| 1 | Halogen, UV light | Free radical substitution |
| 2 | H2, Ni or Pt catalyst, heat | Electrophilic addition |
| 3 | Al₂O₃ catalyst, heat | Thermal decomposition |
| 4 | Hydrogen halide or halogen | Electrophilic addition |
| 5 | NaOH in ethanol, reflux | Elimination |
| 6 | Conc. NH3 in ethanol, heat under pressure | Nucleophilic substitution |
| 7 | KCN in ethanol, reflux | Nucleophilic substitution |
| 8 | Dilute HCl(aq) | Acid-base |
| 9 | NaOH(aq), reflux | Nucleophilic substitution |
| 10 | Hydrogen halide, PCl3 or SOCl2 OR KCl and conc. acid OR PCl5 and heat | Nucleophilic substitution |
| 11 | Steam, H3PO4 catalyst, 300°C, 60 atm | Electrophilic addition |
| 12 | Conc. H2SO4 or Al2O3 catalyst, heat | Elimination |
| 13 | Cold, dilute acidified KMnO₄(aq) | Oxidation |
| 14 | K2Cr2O7(aq) or KMnO4(aq), H2SO4 catalyst, reflux | Oxidation |
| 15 | NaBH4(aq) or LiAlH4 in dry ether, heat | Reduction |
| 16 | K2Cr2O7(aq) or KMnO4(aq), H₂SO₄ catalyst, distill | Oxidation |
| 17 | HCN, KCN catalyst, heat | Nucleophilic addition |
| 18 | Dilute HCl(aq) or dilute NaOH(aq), reflux | Hydrolysis |
| 19 | Carboxylic acid, conc. H2SO4 | Condensation |
| 20 | H2O, dilute HCl(aq), heat | Hydrolysis |
| 21 | LiAlH4 in dry ether, heat | Reduction |
| 22 | Dilute HCl(aq), heat | Hydrolysis |
| 23 | Alcohol, conc. H2SO4 | Condensation |
| 24 | Dilute NaOH(aq) | Acid-base |
| 25 | H2, Ni catalyst, heat OR LiAlH4 in dry ether, heat | Reduction |
| 26 | NH3 or amine | Nucleophilic addition-elimination |
| 27 | SOCl2 or PCl3 OR PCl5 and heat | Substitution |
| 28 | H2O | Nucleophilic addition-elimination |
| 29 | Alcohol | Nucleophilic addition-elimination |
Summary of key organic reactions for aromatic compounds
Below is an overview of the primary types of organic reactions we've discussed for aromatic compounds.
Reactions of benzene
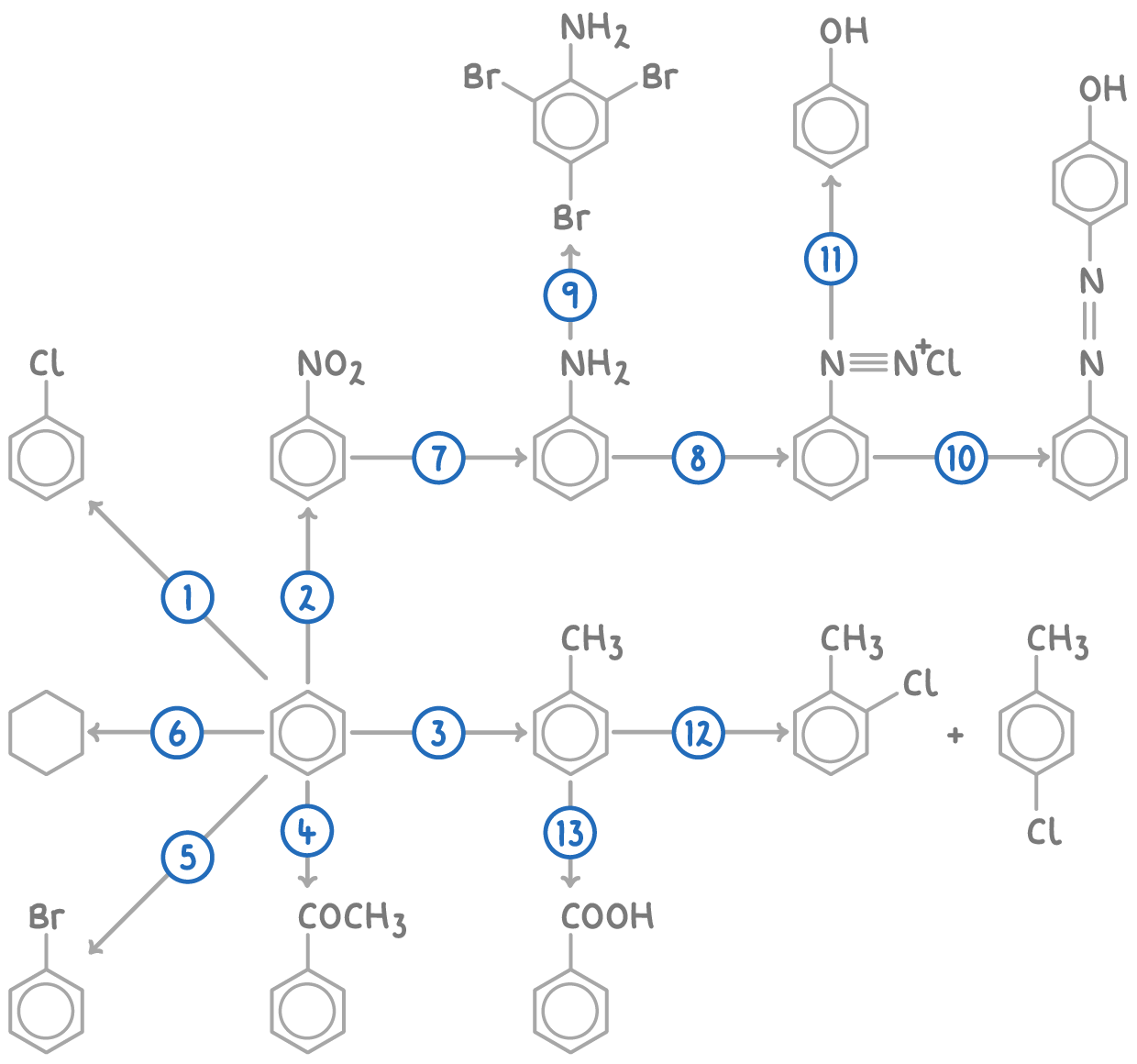
| Reaction | Reagent and conditions | Reaction type |
|---|---|---|
| 1 | Cl2, AlCl3 catalyst | Electrophilic substitution |
| 2 | Conc. HNO3, conc. HCl, 25-60°C | Electrophilic substitution |
| 3 | CH3Cl, AlCl3 catalyst, heat | Friedel-Crafts alkylation |
| 4 | CH3COCl, AlCl3 catalyst, heat | Friedel-Crafts acylation |
| 5 | Br2, AlBr3 catalyst | Electrophilic substitution |
| 6 | H2, Ni or Pt catalyst, heat | Hydrogenation |
| 7 | Sn, conc. HCl, reflux, then NaOH(aq) | Reduction |
| 8 | HNO2(aq) or NaNO2(s) and dilute HCl(aq), below 10°C | Diazotisation |
| 9 | Br2(aq) | Electrophilic substitution |
| 10 | Phenol, NaOH(aq), below 10°C | Electrophilic substitution |
| 11 | H2O(l), warm | Hydrolysis |
| 12 | Cl2, AlCl3 catalyst | Electrophilic substitution |
| 13 | Hot, alkaline KMnO4(aq), dilute acid | Oxidation |
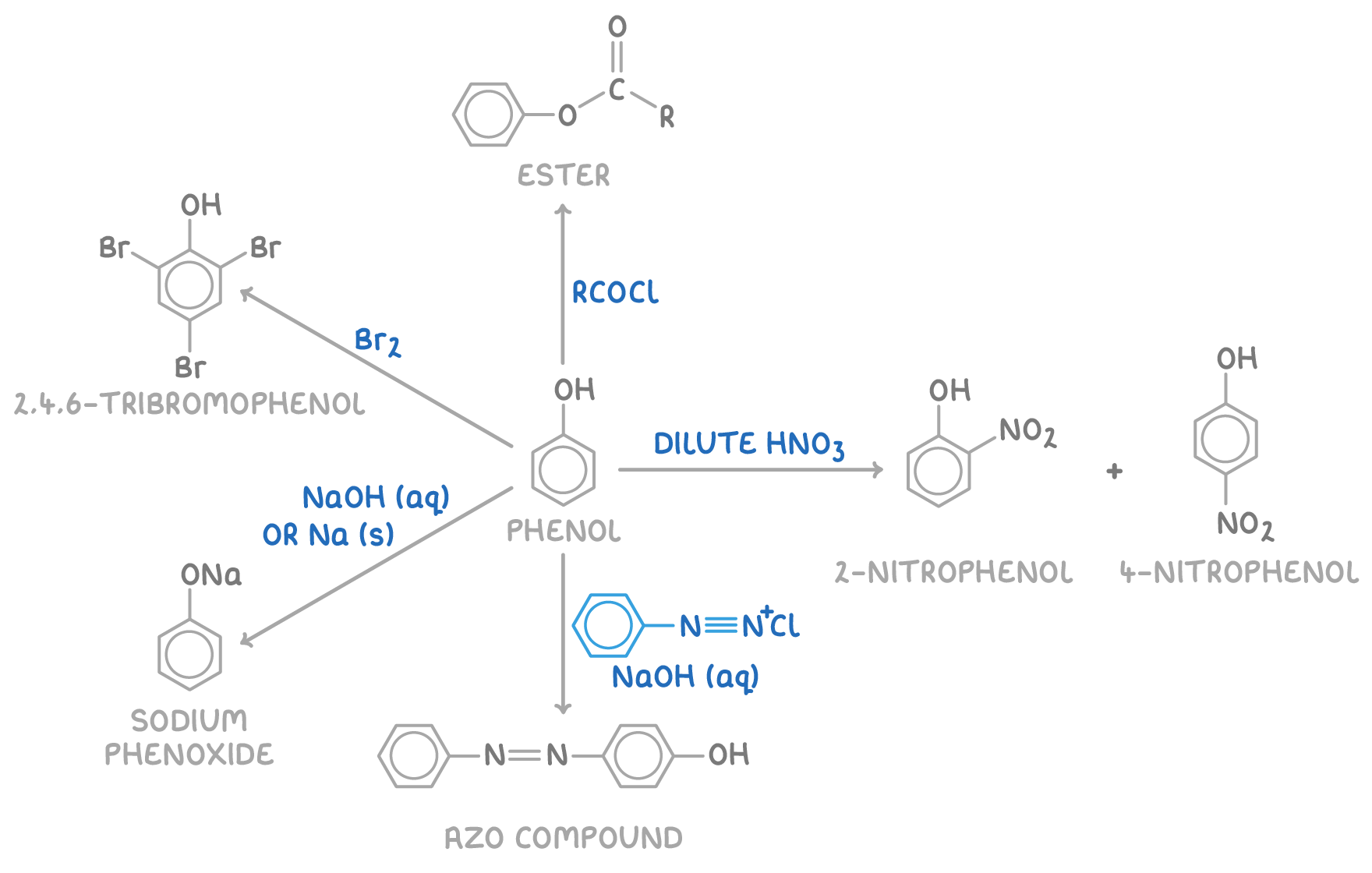
Reactions of phenol
Importance of functional groups
Functional groups are responsible for a molecule's chemical properties and reactivity. Organic molecules are categorised by homologous series based on their functional groups.
Some properties and typical reactions associated with key functional groups are:
| Homologous series | Functional group | Properties | Typical reactions |
|---|---|---|---|
| Halogenoalkane | C-X | Polar bond | Nucleophilic substitution, elimination, Friedel-Crafts alkylation |
| Nitrile | C≡N | Electron deficient C | Reduction, hydrolysis |
| Hydroxynitrile | R2C(OH)C≡N | Electron deficient C | Reduction, hydrolysis |
| Carboxylic acid | -COOH | Electron deficient C | Neutralisation, condensation, reduction, substitution |
| Amine | C–NR2 | Lone pair on nitrogen is basic and can act as a nucleophile | Neutralisation, nucleophilic substitution |
| Amide | RCONHR’ | Electron deficient C | Hydrolysis, reduction |
| Aromatic compounds | C6H5- | Stable delocalised ring of electrons | Electrophilic substitution |
| Acyl chloride | -COCl | Electron deficient C | Nucleophilic addition-elimination, Friedel-Crafts acylation |
So by recognising functional groups, organic chemists can predict the likely behaviour and reactivity of compounds. This allows them to design effective syntheses.