Optical Isomerism
This lesson covers:
- What optical isomers are
- How to identify chiral centres and optical isomers
- How optical isomers rotate plane polarised light
- What racemic mixtures are
- How optical isomers have different biological activities
Optical isomers are mirror images
Optical isomerism is a type of stereoisomerism where isomers have the same structural formula but different arrangements of atoms in space.
A chiral carbon is a carbon with four different groups attached to it. When the groups around a chiral carbon are arranged differently, it results in two non-superimposable mirror image structures called optical isomers.
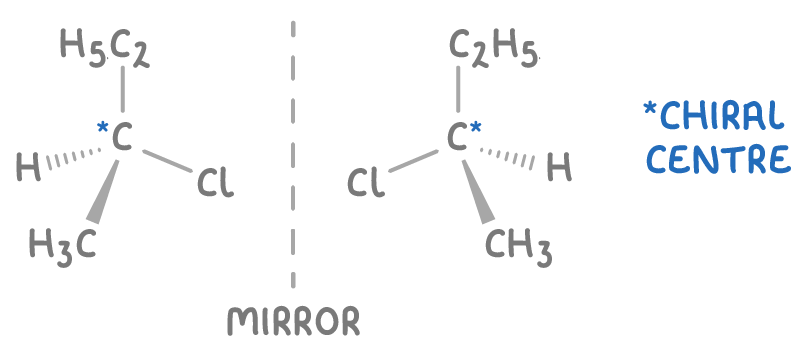
For instance, 2-chlorobutane has a chiral centre on its second carbon, bonded to a chlorine atom, a methyl group, an ethyl group, and a hydrogen atom, resulting in two optical isomers.
To identify and draw optical isomers, follow these steps:
- Identify the chiral carbon - Carefully draw all hydrogen atoms to clearly identify each attachment. Look for the carbon atom connected to four different groups.
- Sketch the optical isomers - Illustrate one enantiomer with its groups arranged tetrahedrally around the chiral carbon. Next, draw its mirror image.
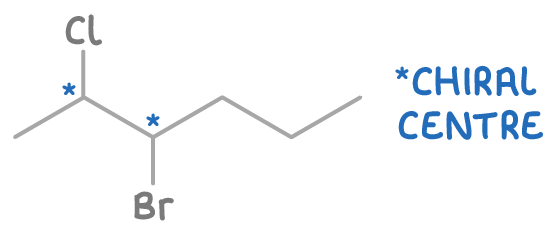
Molecules with multiple chiral centres can have more than two optical isomers, increasing the complexity and number of possible isomers.
For instance, 3-bromo-2-chloropentane has two chiral carbons and hence four optical isomers:
Optical isomers rotate plane polarised light
Optical isomers differ in their effect on plane polarised light:
- Normal light vibrates in all directions while plane polarised light vibrates in one direction only.
- Optical isomers are optically active meaning they rotate plane polarised light.
- One enantiomer rotates plane-polarised light clockwise, while the other enantiomer rotates it anticlockwise by the same angle.
Racemic mixtures contain equal amounts of enantiomers
A racemic mixture, or racemate, is an equimolar mixture containing equal amounts of two enantiomers.
Racemates exhibit no net optical activity as the opposing rotations cancel out.
Enantiomers can have different biological activity
Enantiomers can have different biological activities due to their ability to interact differently with chiral biological molecules, such as proteins and DNA. In some cases, one enantiomer may produce the desired therapeutic effect, while the other enantiomer may be inactive or even toxic. This difference in biological activity highlights the importance of isolating the therapeutic enantiomer from racemic mixtures during pharmaceutical synthesis.
To address this challenge, chiral catalysts have emerged as a valuable tool, offering several advantages in the process of producing single enantiomer drugs:
- Enantioselectivity - Chiral catalysts can selectively produce one desired enantiomer over the other, reducing the formation of unwanted or potentially harmful enantiomers.
- Efficiency - The use of chiral catalysts can improve the efficiency of producing single-enantiomer drugs by increasing the yield and reducing the number of purification steps required.
- Safety - By selectively synthesizing the therapeutic enantiomer, chiral catalysts help minimise the presence of inactive or toxic enantiomers in the final drug product, enhancing patient safety.
- Cost-effectiveness - Chiral catalysts can reduce the overall cost of pharmaceutical production by minimising waste and streamlining the synthesis process.