Covalent Bonding and Simple Molecular Structures
This lesson covers:
- What covalent bonding is
- Exceptions to the 8 electron rule
- Covalent bond strength
- Double and triple covalent bonds
- Dative covalent bonding
Covalent bonding allows atoms to share electrons
A covalent bond is the strong electrostatic attraction formed between a shared pair of electrons and the nuclei of the bonded atoms.
This bonding creates a stable molecule - a group of two or more atoms held together by covalent bonds.
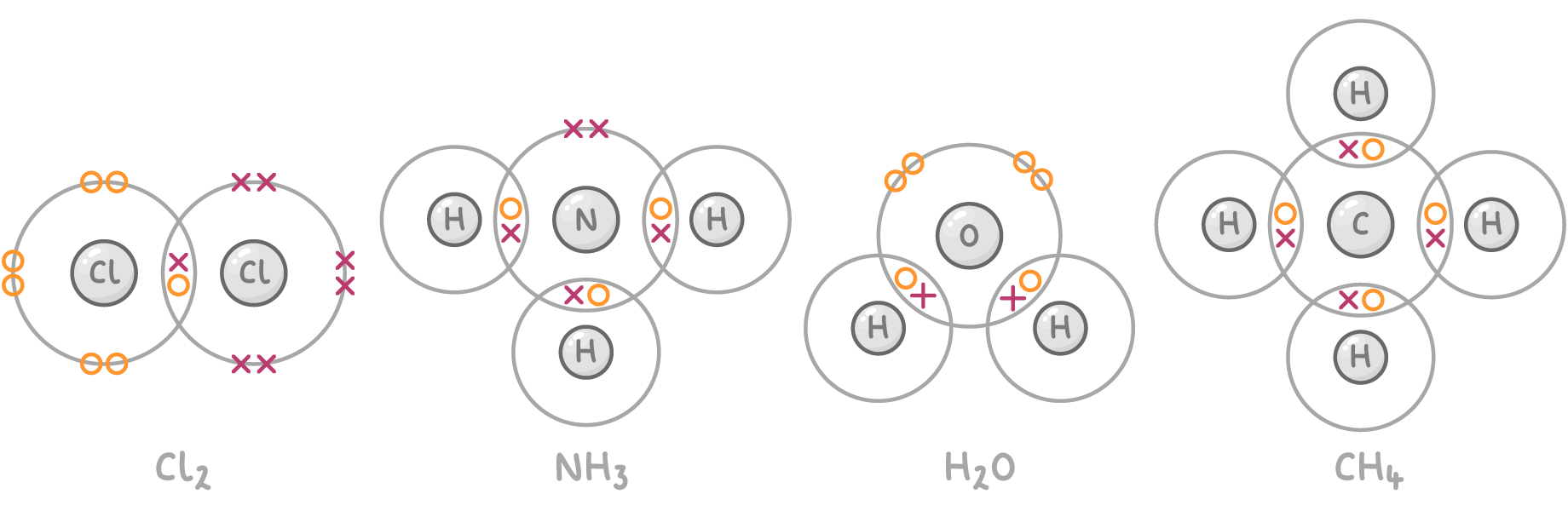
Examples of covalently bonded molecules include:
- Chlorine (Cl2)
- Ammonia (NH3)
- Water (H2O)
- Methane (CH4)
Dot and cross diagrams show how non-metal atoms share electrons to form molecules:
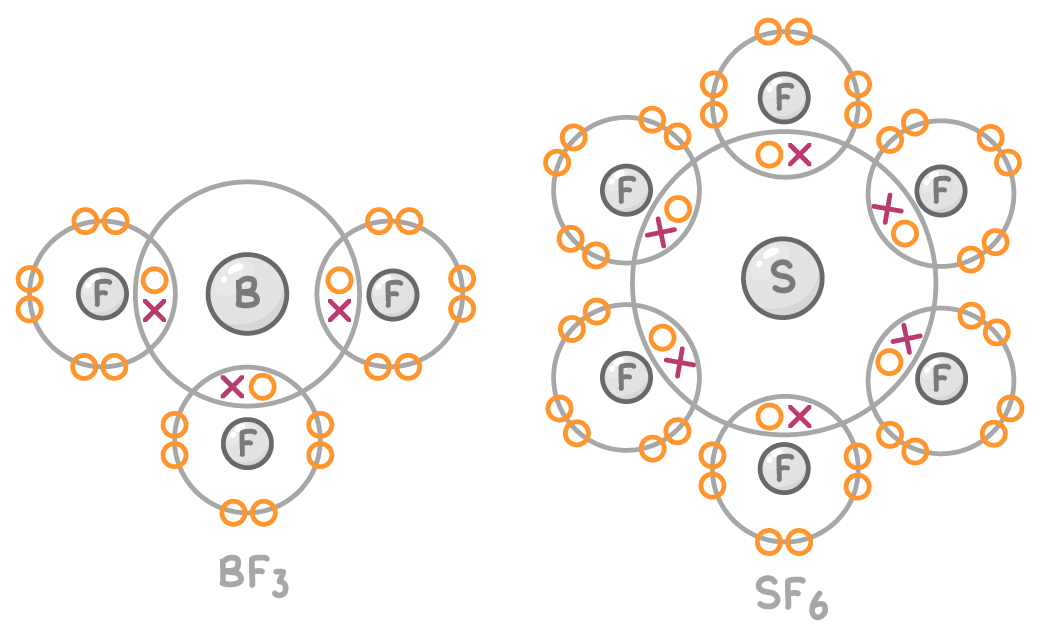
Exceptions to the octet rule
Some exceptions exist where molecules have less or more than 8 outer electrons:
- Boron trifluoride (BF3) has less than 8 electrons (only 6) in its outer shell.
- Sulfur hexafluoride (SF6) has more than 8 electrons (it has 12) in its outer shell.
Covalent bond strength
The strength of a covalent bond depends on how much energy is needed to break the bond.
We measure this bond strength using a value called bond energy, which is the energy required to break one mole of a particular covalent bond in the gaseous state.
The higher the bond energy, the stronger the covalent bond.
For example:
- A N≡N triple bond has a very high bond energy of 945 kJ mol-1, indicating it is a very strong bond.
- In contrast, a F-F single bond has a lower bond energy of 159 kJ mol-1, indicating it is a weaker bond.
Another factor that provides information about the strength of a covalent bond is bond length, which is the internuclear distance between the two covalently bonded atoms. Stronger bonds tend to be shorter in length due to the increased electrostatic attraction between the shared pair(s) of electrons and the nuclei, pulling the atoms closer together.
For example:
- A N≡N triple bond has a very short bond length of 0.109 nm, indicating it is very strong.
- In contrast, a F-F single bond is longer at 0.142 nm, indicating it is a weaker bond.
By considering both bond energies and bond lengths, we can compare the relative reactivity of different covalent molecules. Molecules with longer covalent bonds and lower bond energies, such as F2, tend to be more reactive than molecules with shorter bonds and higher bond energies, such as N2.
Atoms can share multiple pairs of electrons
- The number of covalent bonds an atom forms depends on how many electrons it needs to fill its outer shell.
- Atoms can share multiple electron pairs to fill their outer shell, forming double or triple bonds.
For example:
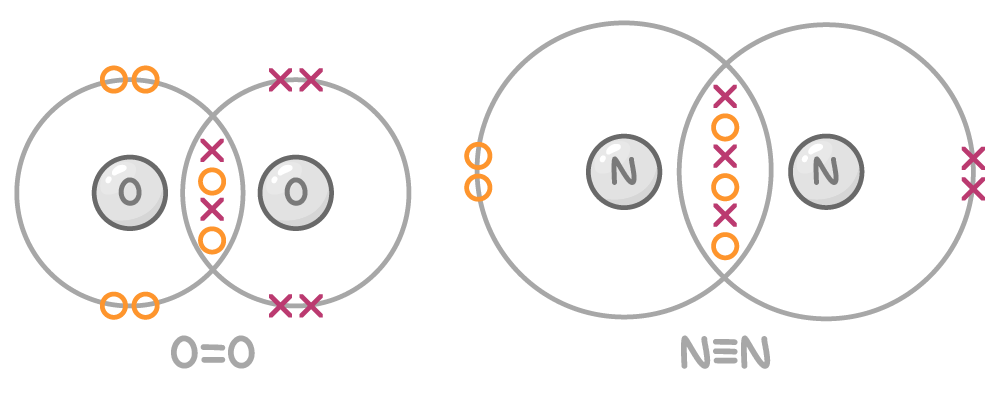
- Oxygen has 6 electrons in its outer shell and needs 2 more to complete it, so it forms a double bond by sharing 2 sets of electrons with another oxygen atom.
- Nitrogen has 5 electrons in its outer shell and needs 3 more to complete it, so it forms a triple bond by sharing 3 sets of electrons with another nitrogen atom.
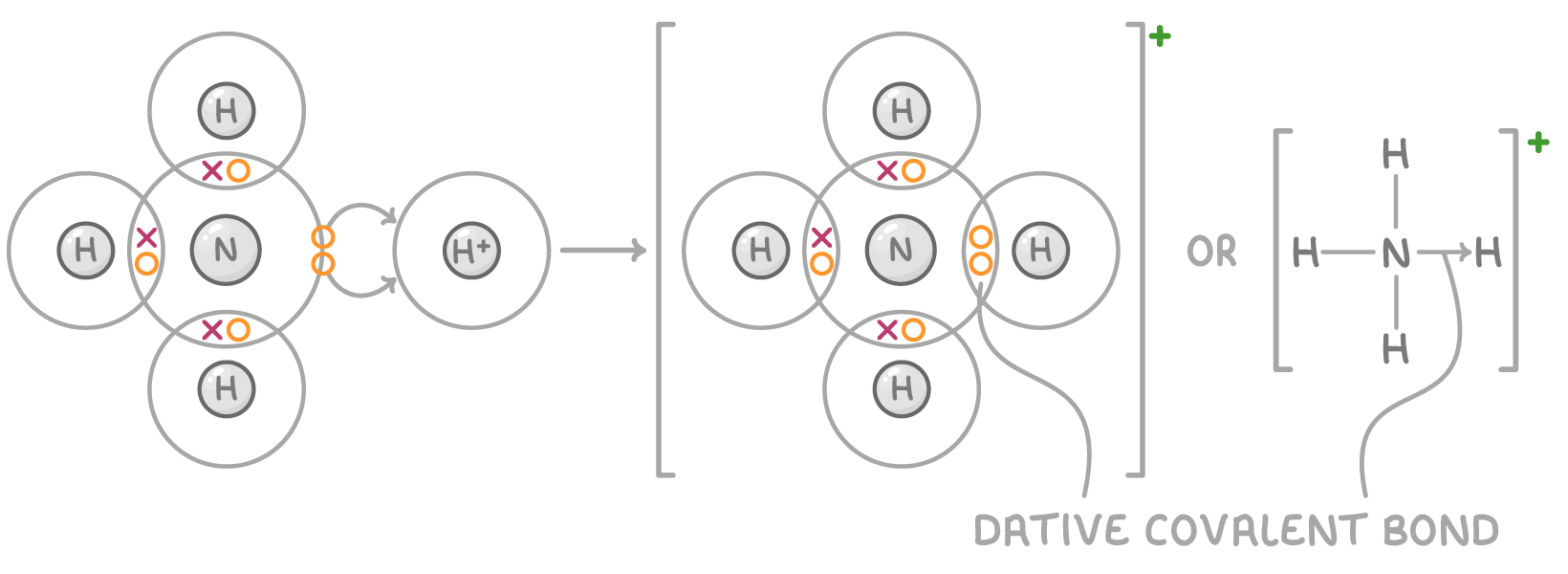
Dative covalent bonding
In dative covalent bonding, also called coordinate bonding, both shared electrons come from just one of the bonding atoms rather than one electron coming from each atom.
For a dative covalent bond to form between two atoms, the following requirements must be met:
- One atom must have a lone pair of electrons to donate.
- The other atom must be electron deficient, (i.e., it must have an incomplete electron shell).
This type of bonding is represented by an arrow showing the direction of electron donation from the atom with the lone pair to the electron-deficient atom.
For example, in ammonium NH4+, the nitrogen atom provides both shared electrons to form a dative covalent bond with the hydrogen ion (H+):