Physical Properties and Reactions of Group 2 Elements
This lesson covers:
- Physical properties of the group 2 elements metals and trends down the group
- Chemical properties relating to the reactivity of the group 2 elements
- Reactions of group 2 elements with water, oxygen, and acids
Melting point generally decreases down group 2
The melting points of the group 2 elements mostly show a decreasing trend down the group, apart from an anomaly at magnesium (Mg).
In their pure elemental form, these metals have a crystalline structure of positive metal ions surrounded by a sea of delocalised electrons.
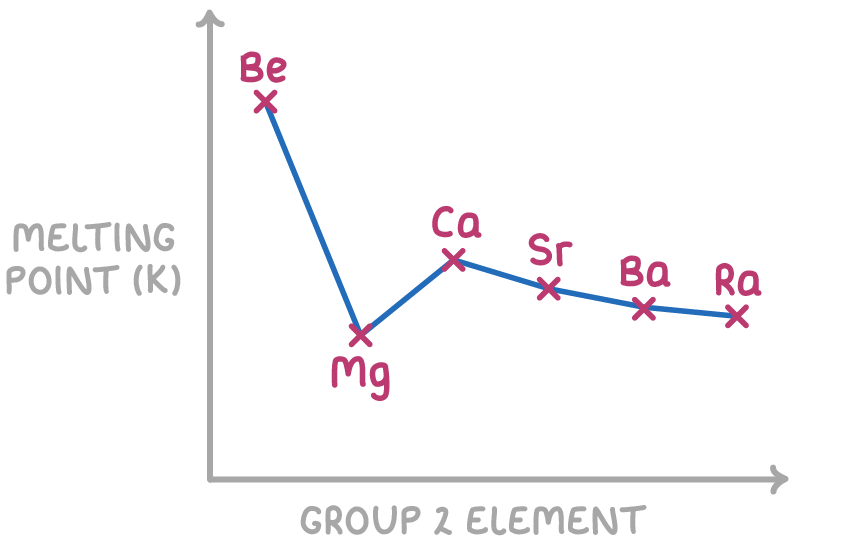
Melting points generally decrease down the group because:
- The ionic radius of the metal ion increases
- This means the delocalised electrons get further away from the positive nuclei
- This results in a weaker electrostatic attraction between the delocalised electrons and the positive nuclei
- So the amount of energy needed to break the metallic bonds decreases
The magnesium anomaly occurs because it forms an unusually stable crystal structure.
Group 2 elements form 2+ ions
The alkaline earth metals readily form 2+ ions when they react, by losing their two outermost electrons.
This can be seen in their electron configurations:
| Element | Electron configuration of atom | Formula of ion | Electronic configuration of ion |
|---|---|---|---|
| Be | 1s2 2s2 | Be2+ | 1s2 |
| Mg | 1s2 2s2 2p6 3s2 | Mg2+ | 1s2 2s2 2p6 |
| Ca | 1s2 2s2 2p6 3s2 3p6 4s2 | Ca2+ | 1s2 2s2 2p6 3s2 3p6 |
The electrons in the outer s sub-shell are relatively weakly bound and are lost during reactions.
This loss of two electrons results in a stable noble gas electron configuration.
First ionisation energy decreases down group 2
Related to atomic radius, the first ionisation energy shows an overall decrease going down the group. This energy is a measure of the strength of electrostatic attraction between the outer electron and the nucleus.
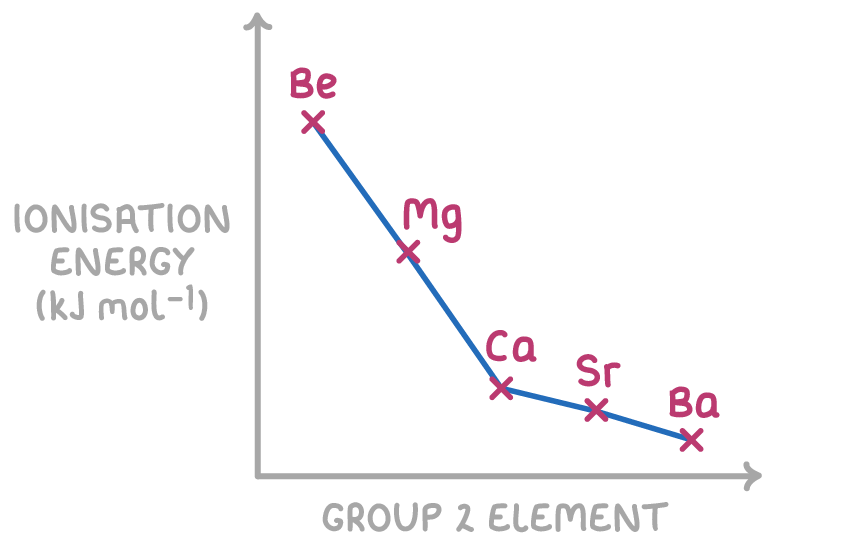
Ionisation energy decreases down group 2 because:
- Nuclear charge - Increases down the group as more protons are added, increasing attraction for electrons
- Atomic radius - Increases down the group as more electron shells are added, moving electrons away from nucleus
- Electron shielding - Increases down group as more inner electron shells reduce nuclear attraction
The atomic radius and shielding effects down groups are greater than the nuclear charge effect, leading to an overall decrease in ionisation energies as you move down group 2.
Reactivity increases down group 2
Since it becomes easier to remove electrons further down the group, the reactivity of group 2 metals increases as you progress from beryllium (Be) down to radium (Ra).
The reactions of group 2 elements with water, oxygen, and acids become more vigorous as you descend the group.
Reaction with water
Reaction of a group 2 metal (M) with cold water produces a metal hydroxide and hydrogen gas:
M(s) + H2O(l) ➔ M(OH)2(aq) + H2(g)
Using calcium as an example:
- Ca(s) + 2H2O(l) ➔ Ca(OH)2(aq) + H2(g)
- Calcium is oxidised from 0 to +2
The reactivity trend of the alkaline earth metals with water is shown below:
| Metal | Reactivity |
|---|---|
| Be | No reaction |
| Mg | Reacts very slowly |
| Ca | Steady reaction rate |
| Sr | Reacts fairly quickly |
| Ba | Rapid reaction |
Reaction with oxygen
When a group 2 metal (M) burns in oxygen gas, a white oxide is formed:
2M(s) + O2(g) ➔ 2MO(s)
Using calcium as an example:
- 2Ca(s) + O2(g) ➔ 2CaO(s)
- Calcium is oxidised from 0 to +2
- Oxygen is reduced from 0 to -2
Reaction with acids
Reaction of a group 2 metal with dilute acids produces a salt and hydrogen gas.
The anion of the salt formed depends on the acid used:
- Hydrochloric acid forms chloride salts
- Sulfuric acid forms sulfate salts
- Nitric acid forms nitrate salts
The table below shows the salt formed and the equations for the reactions of calcium with some common acids. In each example calcium is oxidised from 0 to +2.
| Metal | Acid | Name of salt formed | Balanced symbol equation |
|---|---|---|---|
| Calcium | Hydrochloric acid | Calcium chloride | Ca(s) + 2HCl(aq) ➔ CaCl2(aq) + H2(g) |
| Calcium | Sulfuric acid | Calcium sulfate | Ca(s) + H2SO4(aq) ➔ CaSO4(aq) + H2(g) |
| Calcium | Nitric acid | Calcium nitrate | Ca(s) + 2HNO3(aq) ➔ Ca(NO3)2(aq) + H2(g) |