Giant Molecular Structures and Fullerenes
This lesson covers:
- What giant covalent structures are
- Diamond - bonding, structure, properties
- Graphite - bonding, structure, properties
- Buckminsterfullerene - bonding structure, properties
Giant covalent structures
Some elements can form extensive interconnecting networks of covalently bonded atoms known as giant covalent structures.
- These structures involve huge lattices extending in three dimensions.
- In carbon, the small atomic size and ability to form 4 covalent bonds per atom allow the formation of giant covalent structures.
- The different structural forms of an element in the same state are called allotropes.
There are 3 allotropes of carbon you need to know about:
- Diamond and graphite, which have giant lattice structures
- Buckminsterfullerene. which has a simple molecular structure
Diamond allotrope
Bonding and structure:
Each carbon atom forms 4 very strong covalent bonds with others in a tetrahedral arrangement.
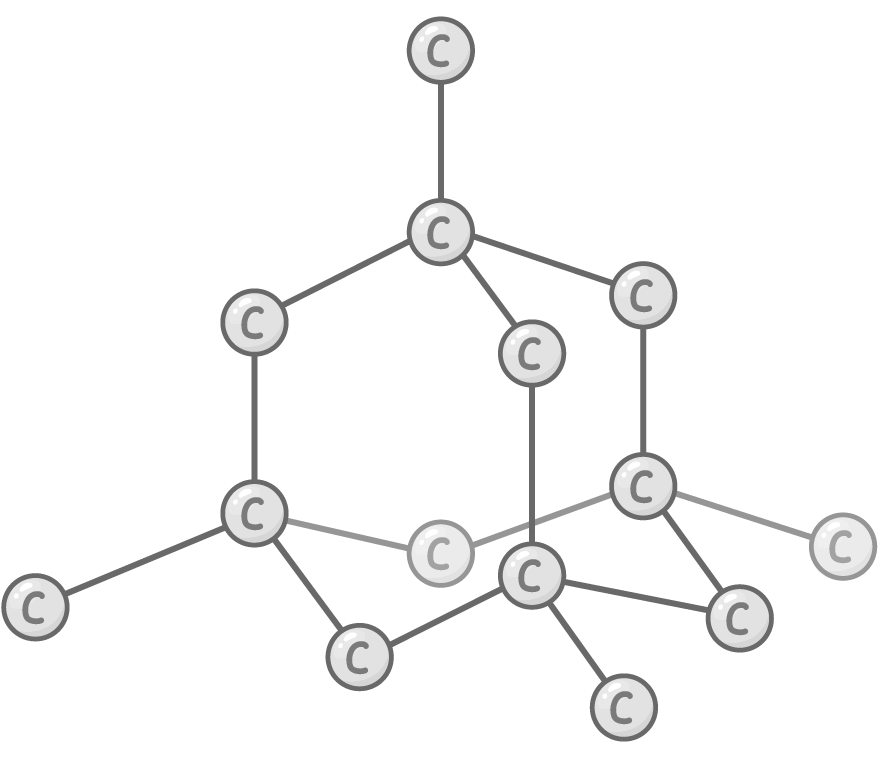
Properties:
- Extremely hard - Extensive network of strong covalent bonds not easily broken
- Very high melting point - Huge amount of energy needed to break enough bonds to melt diamond
- Good thermal conductor - Strong interatomic bonds transmit heat through vibrations
- Electrical insulator - All outer electrons tied up in localised bonds so no free electrons to carry charge
- Insoluble - Covalent bonds too strong to be broken by solvation
Silicon(IV) dioxide (SiO2) is another example of a giant covalent substance; its structure is similar to diamond, with a tetrahedral arrangement around each silicon atom. Like diamond, silicon dioxide forms hard, colourless crystals with high melting and boiling points and it does not conduct electricity.
Graphite allotrope
Bonding and structure:
- Each carbon atom forms 3 strong covalent bonds in a planar hexagonal pattern, with each carbon contributing 1 delocalised electron.
- Multiple stacked layers of hexagonal carbon arrays with weak intermolecular forces between layers.
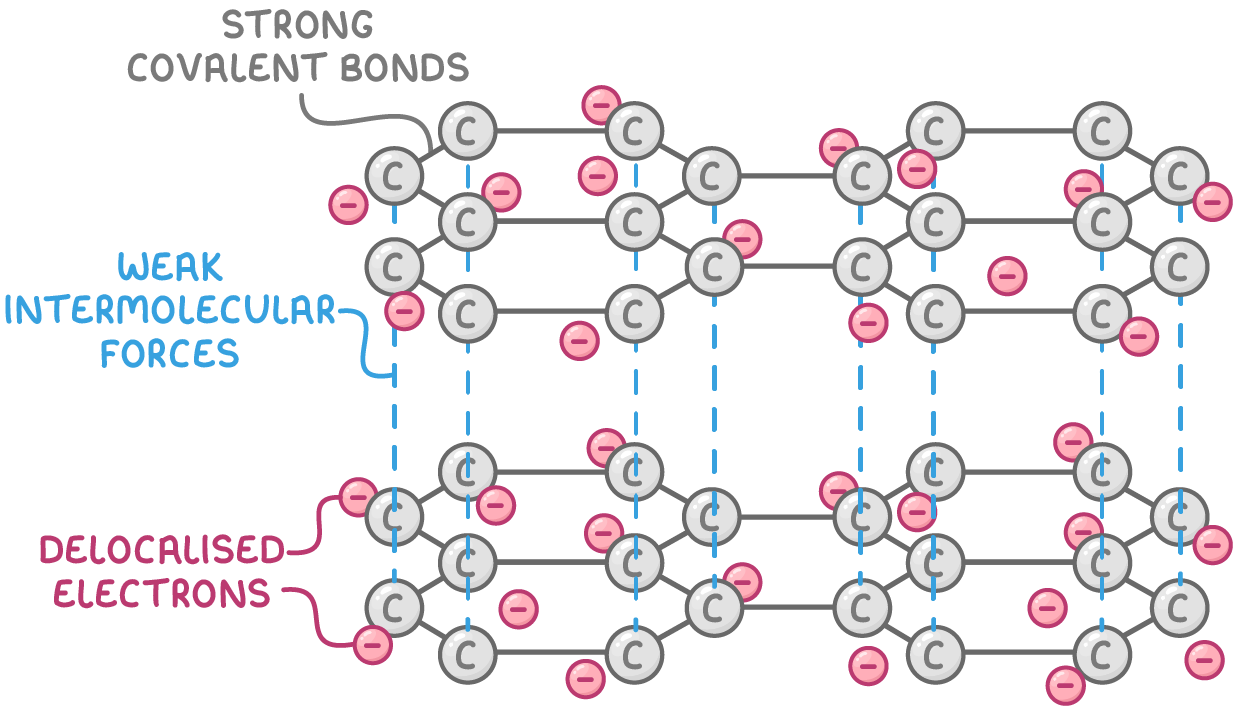
Properties:
- Soft and slippery - Weak intermolecular forces let sheets slide over each other
- Conducts electricity along layers - Delocalised electrons move through the 2D lattice carrying electrical charge
- Lower density than diamond - Weak intermolecular forces lead to increased separation between layers
- High sublimation temperature but lower melting point than diamond - Covalent bonds within each layer are very strong but the weaker intermolecular forces between layers means graphite melts at a lower temperature
Buckminsterfullerene allotrope
Fullerenes are spherical or tubular molecules of carbon atoms arranged in pentagonal and hexagonal rings. The first fullerene to be discovered was Buckminsterfullerene (C60).
Bonding and structure:
- Buckminsterfullerene (C60) has a spherical structure made up of 20 hexagons and 12 pentagons.
- Each carbon atom forms 3 strong covalent bonds, with each carbon contributing 1 delocalised electron.
- Buckminsterfullerene has a simple molecular structure with weak intermolecular forces between spheres.
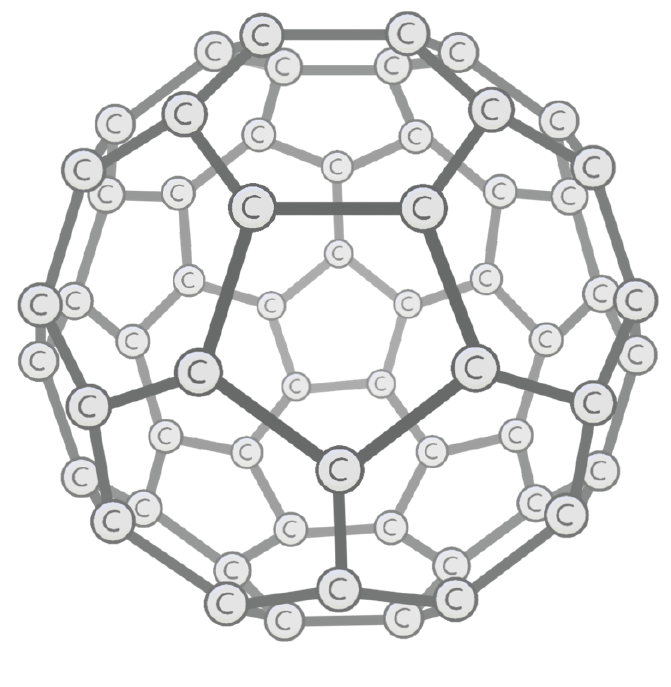
Properties:
- Low sublimation point - Weak intermolecular forces mean less energy is required to directly sublimate from a solid to a gas
- Relatively soft - Little energy needed to overcome the weak intermolecular forces
- Poor electrical conductivity - Reduced electron delocalisation compared to graphite limits conductivity
- Slightly soluble - The spherical shape allows some solubility in certain solvents
- More reactive - Areas of relatively high electron density allow electrophilic reactions
The weak intermolecular forces between the molecules result in these different properties compared to other allotropes.
Summary
The table below summarises the bonding, structure and properties of diamond, graphite, graphene, and Buckminsterfullerene.
| Diamond | Graphite | Buckminsterfullerene | |
|---|---|---|---|
| Bonding | 4 strong 3D covalent bonds per carbon atom in arrangement | 3 strong planar covalent bonds + 1 delocalised electron per carbon. Weaker interlayer forces | 5-6 covalent bonds per carbon atom |
| Structure | 3D network of tetrahedrally bonded carbon atoms | Stacked 2D hexagonal carbon sheets | Spherical structure with 12 pentagons and 20 hexagons |
| Properties | Extremely hard / Very high melting point / Good thermal conductor / Electrical insulator / Insoluble | Softer and layers slide / Conducts electricity in layers / Lower density than diamond / High sublimation temperature | Poor electrical conductivity / Low sublimation point / Insoluble in organic solvents |