Formation of Amines
This lesson covers:
- How aliphatic amines are produced
- The reaction mechanism involved in producing aliphatic amines
Aliphatic amines form from halogenoalkanes, nitriles or amides
There are two main ways to produce aliphatic amines:
- Nucleophilic substitution of halogenoalkanes
- Reduction of nitriles or amides
Producing primary and secondary aliphatic amines
Primary and secondary aliphatic amines can be produced through several reactions.
Halogenoalkanes with ammonia
Aliphatic amines are produced by heating a halogenoalkane under pressure with excess ammonia in ethanol.
For example, ethylamine is produced from bromoethane:
CH3CH2Br + 2NH3 ➔ CH3CH2NH2 + NH4Br
This involves nucleophilic substitution via a two-step mechanism:
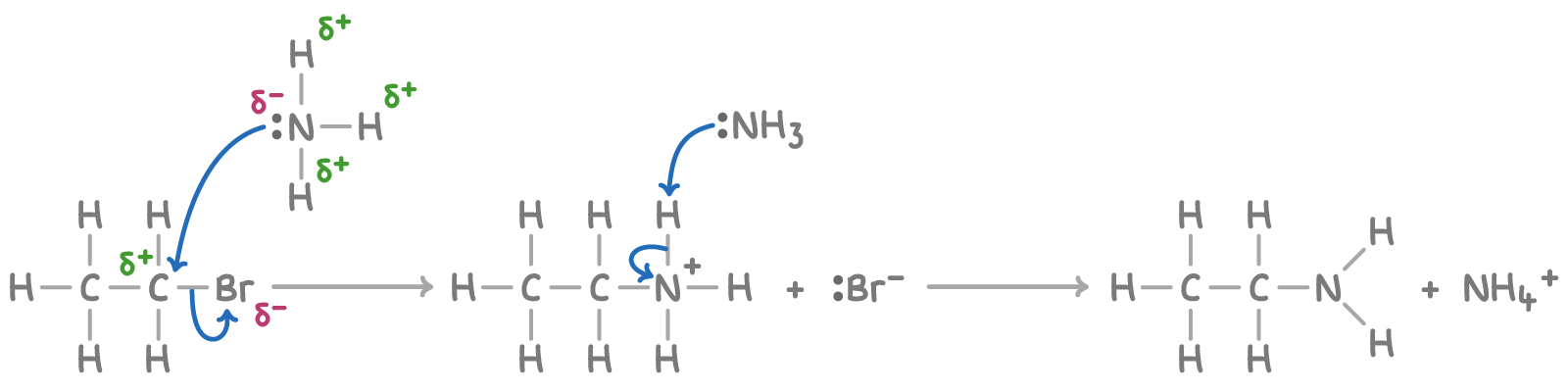
- The halogenoalkane is attacked by ammonia, displacing the halogen and forming an alkylammonium salt.
- A second ammonia molecule then removes a proton from the salt to form the amine product.
Halogenoalkanes with primary amines
Heating a halogenoalkane under pressure with a primary amine in ethanol in a sealed tube yields secondary amines through nucleophilic substitution.
For example, diethylamine is produced from bromoethane:
CH3CH2Br + 2CH3CH2NH2 ➔ (CH3CH2)2NH + HBr
Reducing amides with LiAlH4
Amides can be reduced by lithium aluminium hydride (LiAlH4) to produce primary amines:
RCONH2 + 4[H] ➔ RCH2NH2 + H2O
Reducing nitriles
Nitriles are reduced to primary amines using lithium aluminium hydride (LiAlH4) or catalytic hydrogenation with H2 and Ni catalyst:
R-C≡N + 4[H] ➔ R-CH2-NH2