Primary Amines, Nitriles & Hydroxynitriles
This lesson covers:
- How amines are produced from halogenoalkanes
- How nitriles are produced from halogenoalkanes
- How hydroxynitriles are produced from carbonyl compounds
- The acid hydrolysis of nitriles to carboxylic acids
Producing amines
Amines (RNH2) can be produced by reacting halogenoalkanes with ammonia (NH3).
Heating a halogenoalkane under pressure with excess concentrated NH3 in ethanol allows nucleophilic substitution to occur, displacing the halogen and producing a primary amine.
For example, reacting bromoethane with ammonia yields ethylamine:
CH3CH2Br + 2NH3 ➔ CH3CH2NH2 + NH4Br
This reaction proceeds via a two-step mechanism:
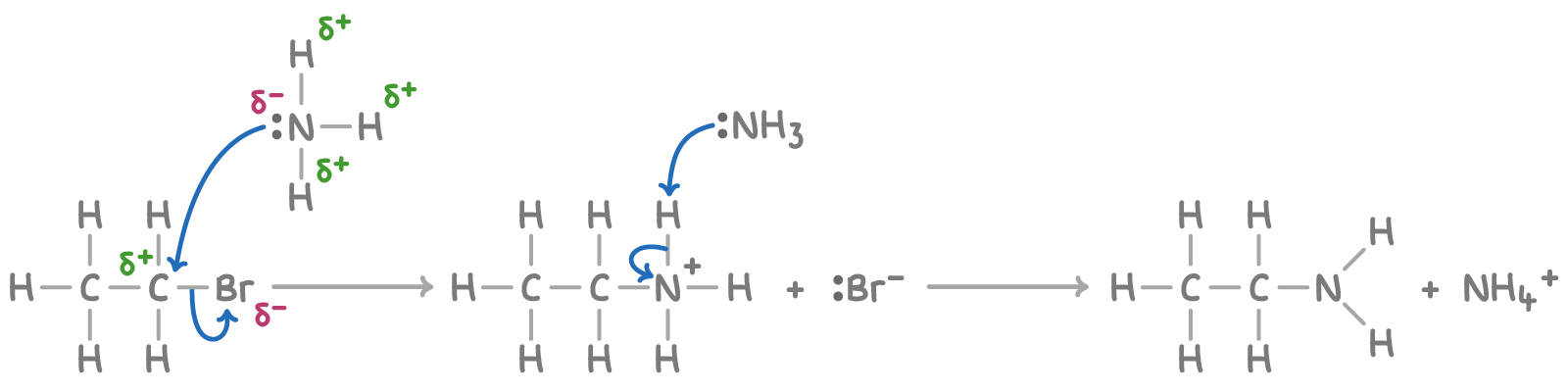
- The halogenoalkane is attacked by ammonia, displacing the halogen and forming an alkylammonium salt.
- A second ammonia molecule then removes a proton from the salt to form the amine product.
Producing nitriles
Nitriles (RC≡N) can be produced by the substitution reaction of halogenoalkanes with cyanide ions (CN-).
An ethanolic potassium cyanide (KCN) solution provides the cyanide ions. When heated under reflux with a halogenoalkane, the CN- acts as a nucleophile and substitutes for the halogen.
For example, bromoethane reacts with CN- to form propanenitrile:
CH3CH2Br + CN- ➔ CH3CH2C≡N + Br-
This nucleophilic substitution reaction proceeds via a one-step SN2 nucleophilic substitution mechanism:
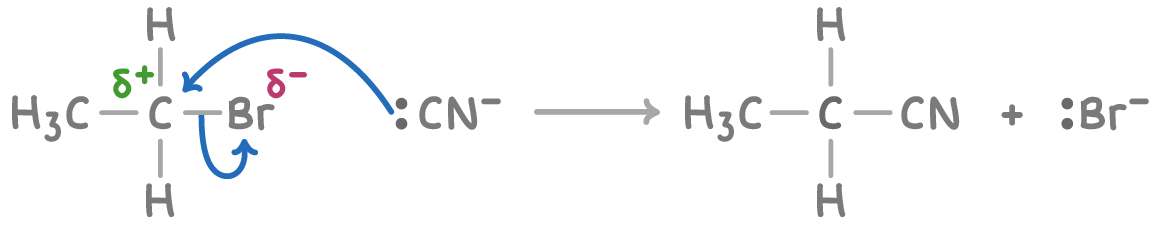
Notice that this reaction adds an extra carbon atom to the halogenoalkane's original chain. So it can be used when chemists need to extend the carbon chain length of an organic starting material.
Producing hydroxynitriles
Aldehydes and ketones undergo nucleophilic addition reactions with hydrogen cyanide (HCN) across the C=O double bond.
In this reaction, a nitrile (-C≡N) group is added to the carbonyl carbon, increasing the carbon chain length by one carbon.
For example, propanal reacts with HCN to form 2-hydroxybutanenitrile:
CH3CH2CHO + HCN ➔ CH3CH2CH(OH)CN
The HCN is produced in situ by the reaction of KCN and dilute H2SO4. Heat is required to initiate the reaction.
This reaction proceeds via a two-step mechanism:
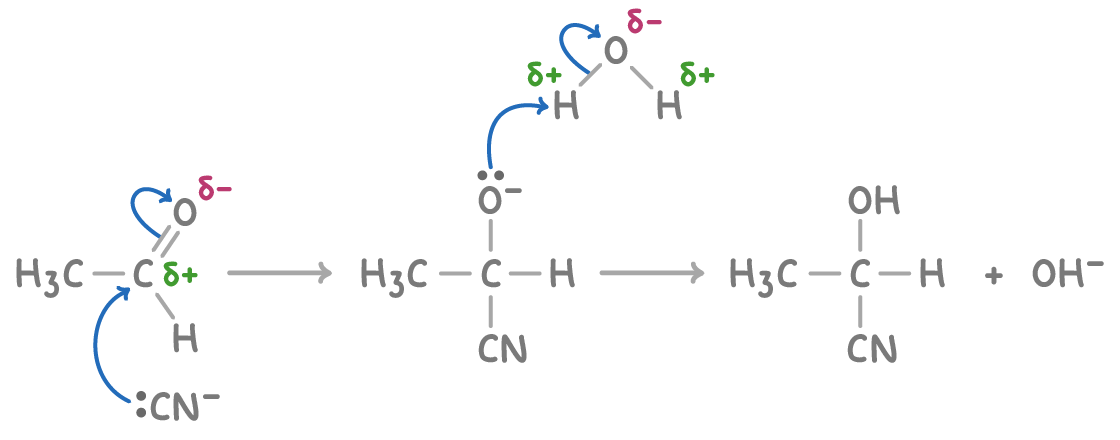
- The polarised C=O bond of the carbonyl compound is attacked by the cyanide anion (CN-), which acts as a nucleophile. This attack occurs at the positive carbon of the C=O bond, forming an unstable negatively charged intermediate.
- The negatively charged intermediate rapidly reacts with a proton (H+) from the HCN, dilute acid, or water. This protonation gives the final hydroxynitrile product.
Hydrolysing nitriles to carboxylic acids
The nitrile group (-C≡N) can be easily hydrolysed to yield a carboxylic acid (-COOH).
There are two methods to carry out nitrile hydrolysis:
- Refluxing with dilute hydrochloric acid.
- Refluxing with dilute sodium hydroxide then acidifying.
Acidic hydrolysis
When a nitrile is refluxed with dilute HCl, the nitrile group is converted to a carboxylic acid:
RC≡N + HCl + 2H2O ➔ RCOOH + NH4Cl
For example, ethanenitrile hydrolyses to ethanoic acid:
CH3C≡N + 2H2O ➔ CH3CH2COOH + NH4Cl
Alkaline hydrolysis
Refluxing a nitrile with dilute NaOH produces the sodium salt of the carboxylic acid:
RC≡N + NaOH ➔ RCOONa + NH3
To obtain the carboxylic acid, the salt is treated with a strong acid like dilute H2SO4:
RCOONa + H2SO4 ➔ RCOOH + NaHSO4
For example, ethanenitrile is hydrolysed stepwise:
CH3C≡N + NaOH ➔ CH3COONa + NH3
2CH3COONa + H2SO4 ➔ 2CH3COOH + Na2SO4