Bonding in Organic Molecules
This lesson covers:
- How to describe the shapes of organic molecules (straight-chained, branched, or cyclic)
- Hybridisation of carbon atoms (sp, sp2, sp3) and effects on bond angles
- Sigma (σ) and pi (π) bonds formed by carbon atoms
- The concept of planar molecule shapes
Organic molecule shapes
Organic molecules containing carbon can have different overall shapes:
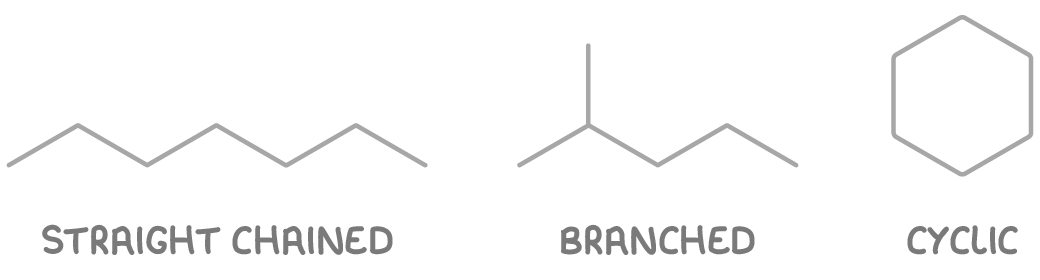
- Straight-chained - Carbon atoms form a linear chain structure, for example, alkanes like heptane (CH3(CH2)5CH3).
- Branched - Side chains of carbon atoms are attached to the main chain, for example, 2-methylpentane (CH3CH(CH3)CH2CH2CH3).
- Cyclic - The carbon backbone forms a ring structure with no end carbon atoms, for example, cyclohexane (C6H12).
Hybridisation of carbon atoms
Orbital hybridisation is the process in which atomic orbitals combine and reshuffle their characteristics to form new hybrid orbitals. This allows atoms such as carbon to form stronger and more directional covalent bonds.
Carbon commonly hybridises its 2s and 2p atomic orbitals to form stronger bonds:
- sp hybridisation - 1 s orbital + 1 p orbital. Seen in alkynes with C≡C triple bonds like ethyne (HC≡CH).
- sp2 hybridisation - 1 s orbital + 2 p orbitals . Seen in alkenes with C=C double bonds like ethene (H2C=CH2).
- sp3 hybridisation - 1 s orbital + 3 p orbitals. Seen in alkanes with C-C single bonds like ethane (H3C-CH3).
Hybridisation affects the geometry and angles between bonds:
| Type | Bonds | Angle (°) | Shape | Example |
|---|---|---|---|---|
| sp | 2 σ bonds + 2 π bonds | 180 | Linear | HC≡CH |
| sp2 | 3 σ bonds + 1 π bond | 120 | Trigonal planar | H2C=CH2 |
| sp3 | 4 σ bonds | 109.5 | Tetrahedral | H3C-CH3 |
Sigma and pi bonds in organic molecules
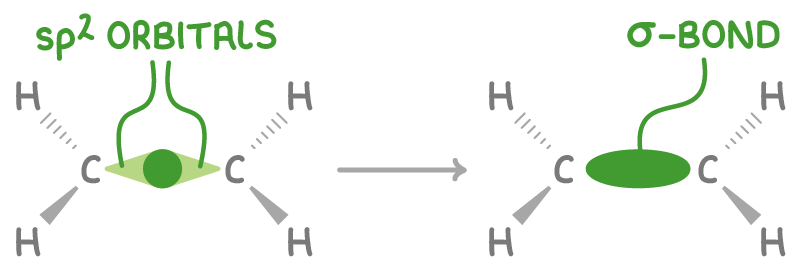
Sigma (σ) bonds involve head-on overlap between atomic orbitals, forming very strong single covalent bonds. Carbon forms 4 σ bonds.
For example, the sp2 orbitals of the two carbon atoms in ethene (C2H4) overlap directly to form a C-C sigma bond.
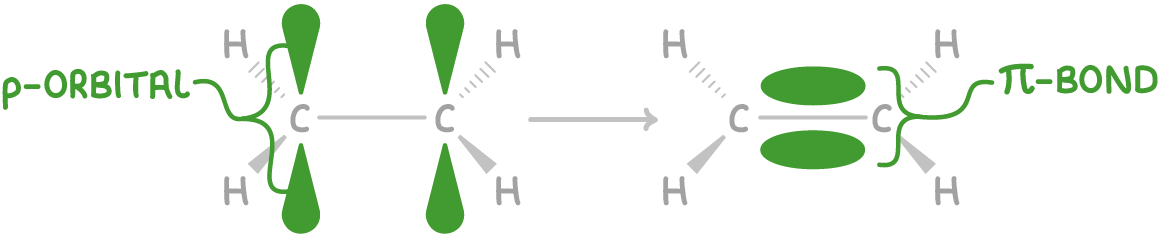
Pi (π) bonds involve sideways overlap of atomic orbitals, above and below the plane of the molecule. They are weaker than sigma bonds.
Carbon forms double bonds (1 σ bond + 1 π bond) and triple bonds (1 σ bond + 2 π bonds).
For example, the p orbitals of the two carbon atoms in ethene (C2H4) overlap sideways to create a C-C π bond, forming an overall C=C double bond.
Planar shapes in organic molecules
Molecules with carbon-carbon double bonds like ethene (H2C=CH2) are described as planar.
This means all the atoms lie in the same flat plane, with bond angles of about 120° due to sp2 hybridisation of the carbon atoms.
Planarity maximises orbital overlap during pi bond formation.