Reactions of the Amides
This lesson covers:
- Amides as carboxylic acid derivatives
- Why amides are weaker bases than amines
- Making amides from acyl chlorides
- The mechanism of amide formation
- Reactions of amides
Amides are carboxylic acid derivatives
Amides are organic compounds that contain the functional group –CONH2. They are derived from carboxylic acids by replacing the hydroxyl group with an amino group.
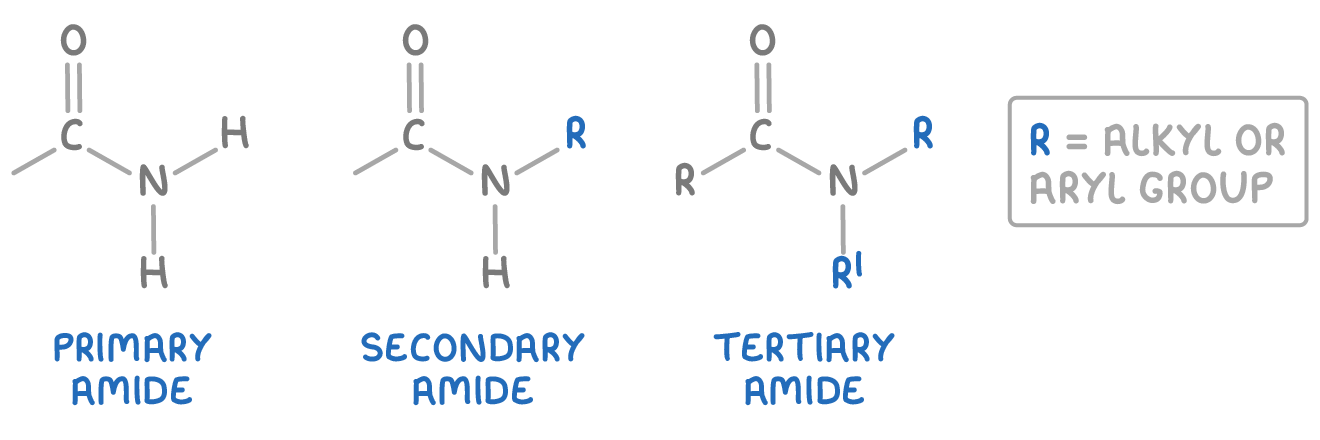
There are three main types of amide you should be familiar with:
- Primary amides - These are amides where the nitrogen atom is bonded to one carbonyl group (C=O) and two hydrogen atoms.
- Secondary amides - These are amides where the nitrogen atom is bonded to one carbonyl group (C=O), one hydrogen atom and one alkyl or aryl group.
- Tertiary amides - These are amides where the nitrogen atom is bonded to one carbonyl group (C=O), and two alkyl or aryl groups.
Amides are much weaker bases than amines
Amides like RCONH2 are significantly weaker bases than amines such as RNH2.
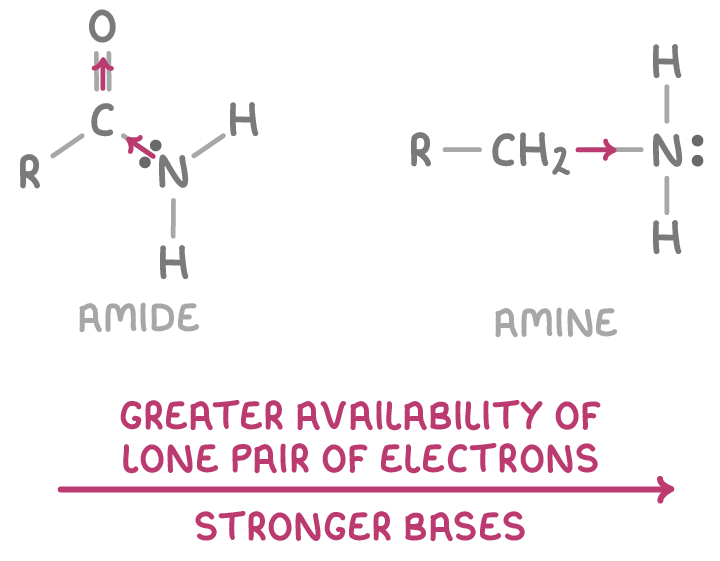
This difference arises because:
- In amides, the carbonyl group delocalises the nitrogen lone pair electrons into the carbonyl π-system through resonance.
- This delocalisation reduces the electron density on the amide nitrogen and diminishes the availability of its lone pair.
- Therefore, the amide nitrogen has a reduced ability to accept H+ protons, making amides much weaker Brønsted-Lowry bases compared to amines, especially in aqueous conditions.
- Amines lack this carbonyl electron-withdrawing effect, retaining lone pair availability and more basicity.
Making amides from acyl chlorides
Amides can be synthesised through the reaction of acyl chlorides with concentrated ammonia or primary amines, typically occurring at room temperature.
With ammonia:
The reaction between acyl chlorides and ammonia produces primary amides.
For example, ethanoyl chloride reacts with ammonia to give ethanamide and HCl:
CH3COCl + NH3 ➔ CH3CONH2 + HCl
With amines:
The reaction between acyl chlorides and primary amines produces secondary amides, also known as N-substituted amides.
For example, ethanoyl chloride reacts with methylamine to give N-methylethanamide and HCl:
CH3COCl + CH3NH2 ➔ CH3CONHCH3 + HCl
In these reactions, the produced HCl typically reacts with any excess ammonia or amine to form ammonium salts.
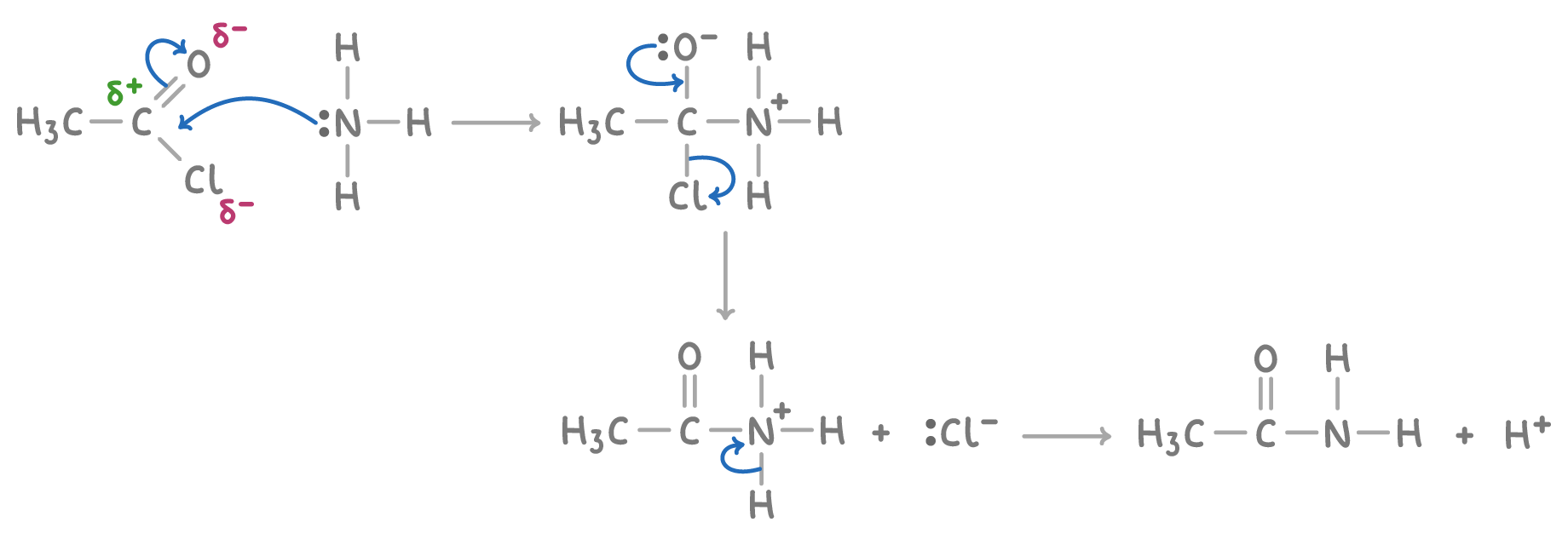
Mechanism of amide formation
The formation of amides through this method involves a two-step nucleophilic addition-elimination mechanism.
Step 1 - Nucleophilic addition
- The nitrogen's lone pair in ammonia or an amine attacks the electrophilic carbon in the carbonyl group of the acyl chloride, leading to a tetrahedral intermediate.
Step 2 - Elimination
- The next step is the elimination of HCl from the intermediate, which re-establishes the carbonyl group and results in the formation of the amide product.
For example, the mechanism for the reaction between ethanoyl chloride and ammonia is:
Reactions of amides
Hydrolysis of amides
Amides feature a characteristic –CONH– linkage, which can be broken through hydrolysis, either by using acids or bases.
Acid-catalysed hydrolysis:
- By refluxing an amide with an acid such as HCl or H2SO4, the C-N bond breaks.
- For primary and secondary amides, the products are a carboxylic acid and an ammonium salt
- For example, the hydrolysis of ethanamide using excess HCl produces ethanoic acid ammonium chloride:
CH3CONH2 + H2O + HCl ➔ CH3COOH + NH4Cl
Base-catalysed hydrolysis:
- When an amide is refluxed with a base like NaOH or KOH, the C-N bond breaks.
- For secondary amides, the products are a carboxylate salt and a primary amine.
- For primary amides, the products are a carboxylate salt and ammonia.
- For exampe, the hydrolysis of ethanamide using NaOH produces sodium ethanoate and ammonia:
CH3CONH2 + NaOH ➔ CH3COONa + NH3
Reduction of amides
Amides can also be reduced to amines using lithium aluminium hydride (LiAlH4) in dry ether.
For example, LiAlH4 reduces ethanamide to ethylamine:
CH3CONH2 + 4[H] ➔ CH3CH2NH2 + H2O
In this reaction, the carbonyl group is reduced to an alcohol group, which subsequently eliminates water to yield the amine product.
This process is particularly useful for converting amides into amines for further synthesis.