Periodicity of Physical and Chemical Properties
This lesson covers:
- How bond strength affects melting and boiling points across period 3
- Trends in atomic radius, ionic radius and electrical conductivity across period 3
Melting and boiling points reflect structure and bonding across period 3
The type of bonds and strength of bonds between atoms affects the melting and boiling points of elements across period 3.
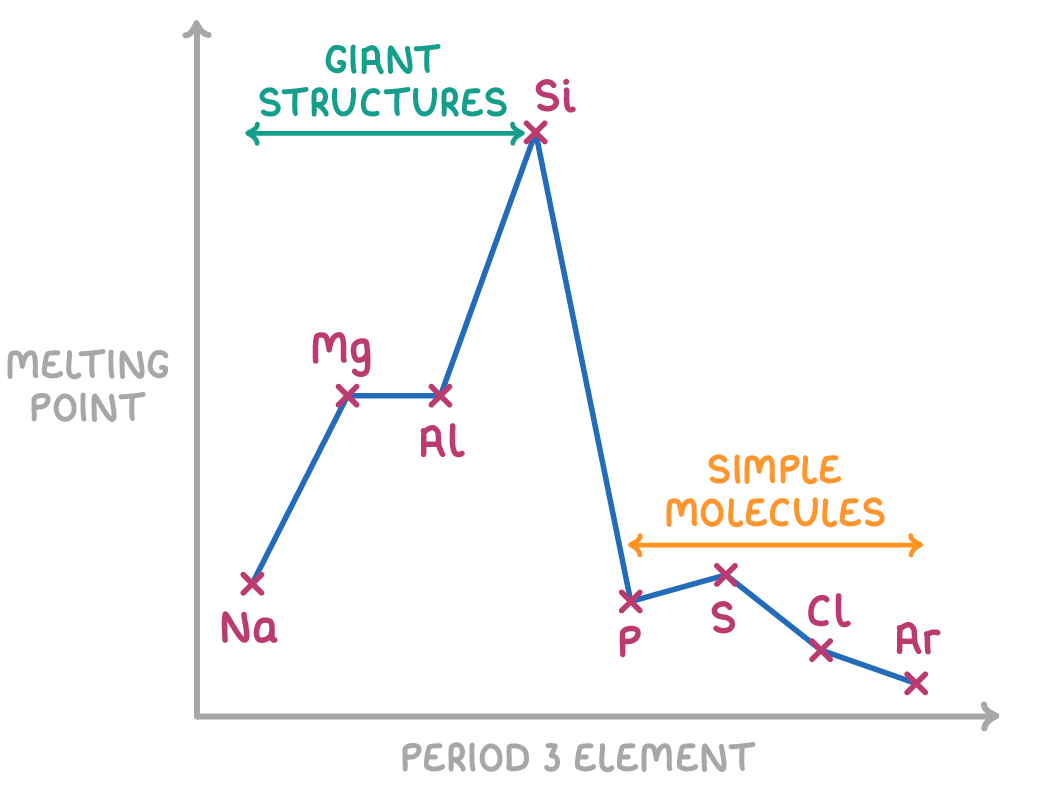
- For the metals sodium (Na), magnesium (Mg) and aluminium (Al), the melting and boiling points increase across the period due the increasing strength of the metallic bonding.
The strength of metallic bonding increases across the period because:
- The ions have a larger positive charge (1+, 2+, 3+).
- The ions have a smaller ionic radius.
- There will be more delocalised electrons (e.g. Na only has 1 delocalised electron per ion, but Al has 3 per ion).
- These three factors all result in a stronger electrostatic attraction between metal cations and delocalised electrons which requires increasing amounts of energy to overcome.
- Silicon (Si) has giant covalent lattice structure. Here, each atom is covalently bonded to 4 neighboring atoms in a tetrahedral arrangement. This forms very strong bonds linking all the atoms together in a 3D lattice. A huge amount of energy is required to break these covalent bonds, resulting in an extremely high melting and boiling point.
- Phosphorus (P4), sulfur (S8), and chlorine (Cl2) have simple molecular structures, with only weak induced dipole-dipole forces existing between the molecules. These intermolecular forces are easily overcome, requiring little energy, and so they result in low melting and boiling points.
- Sulfur (S8) has the highest melting and boiling points among the three because its molecules contain more electrons than phosphorus and chlorine. The larger S8 molecules allow stronger induced dipole-dipole forces to form between molecules.
- Chlorine (Cl2) has the lowest melting and boiling points among the three because its molecules contain the fewest electrons, resulting in the weakest intermolecular forces between Cl2 molecules.
- The noble gas argon (Ar) has the lowest melting and boiling point. Its atoms do not form any bonds and only very weak induced dipole-dipole forces attract them. Minimal energy is needed to overcome these negligible forces between atoms.
Atomic radius decreases across period 3
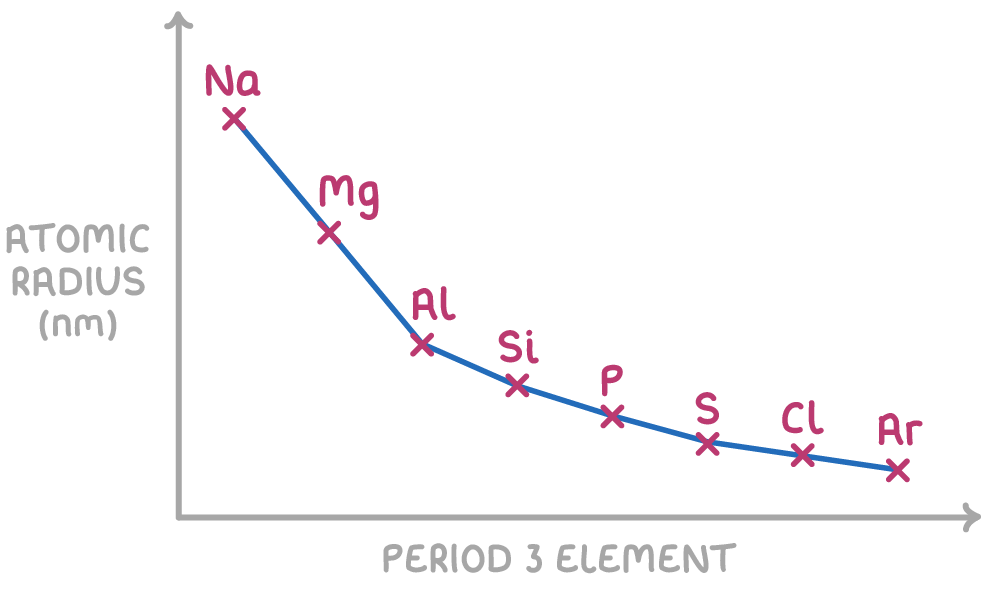
Atomic radius decreases across a period because:
- As protons are added across a period, the nuclear charge increases. This results in a stronger electrostatic attraction between the nucleus and the outer electrons, drawing the outer electrons closer to the nucleus.
- The electrons added across a period go into the outer energy level, so provide little additional shielding for inner electrons.
- With increasing nuclear charge and minimal change in shielding, the stronger electrostatic attraction causes the atomic radius to decrease across the period.
Trends in ionic radius across period 3
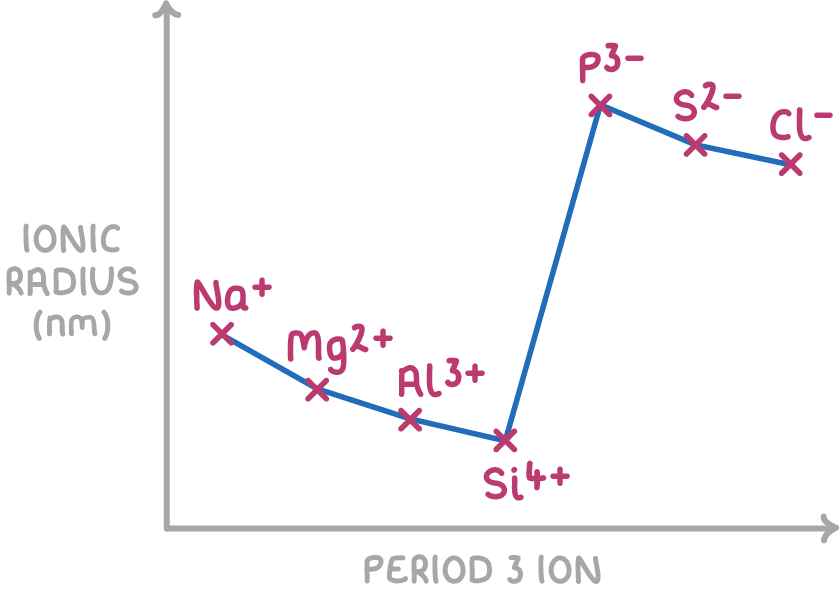
Across a period, the ionic radius decreases for cations but increases for anions because:
- For positive ions (cations), the ionic radius decreases across a period as nuclear charge increases. With more protons, the electrostatic attraction between the nucleus the remaining electrons increases, drawing the remaining electrons closer to the nucleus.
- For negative ions (anions), the ionic radius increases across a period as the addition of electrons to the outer energy level results in more shielding. This weaker electrostatic attraction allows the outer electrons to locate further from the nucleus.
- Anions have larger ionic radii than cations from the same period because they have an extra electron shell; the increase in shielding outweighs the increase in nuclear charge.
Electrical conductivity increases from sodium to aluminium
The electrical conductivity of metals increases from sodium to aluminium across period 3. This trend can be explained by the increasing number of delocalised electrons per metal atom.
- Sodium (Na) has 1 delocalised electron per atom
- Magnesium (Mg) has 2 delocalised electrons per atom
- Aluminium (Al) has 3 delocalised electrons per atom
As the number of delocalised electrons per atom increases, there are more charge carriers available to conduct electricity through the metal lattice. This results in a higher electrical conductivity.
The remaining elements of period 3, from silicon to argon, do not conduct electricity due to the absence of delocalised electrons in their structures.