Acyl Chlorides
This lesson covers:
- The structure and nomenclature of acyl chlorides
- How acyl chlorides are made from carboxylic acids
- Reactions of acyl chlorides
- The mechanism of acyl chloride reactions
- Relative ease of acyl chloride hydrolysis
Acyl chlorides contain the functional group -COCl
Acyl chlorides, also known as acid chlorides, are a type of compound that features the functional group -COCl. These compounds are derived from carboxylic acids, where the hydroxyl (-OH) group is replaced by a chlorine atom (-Cl).
Similar to aacyl chlorides, acid anhydrides and amides are also derivatives of carboxylic acids.

To name an acyl chloride, the suffix "-oic acid" of the parent carboxylic acid is replaced with "-oyl chloride". For example:
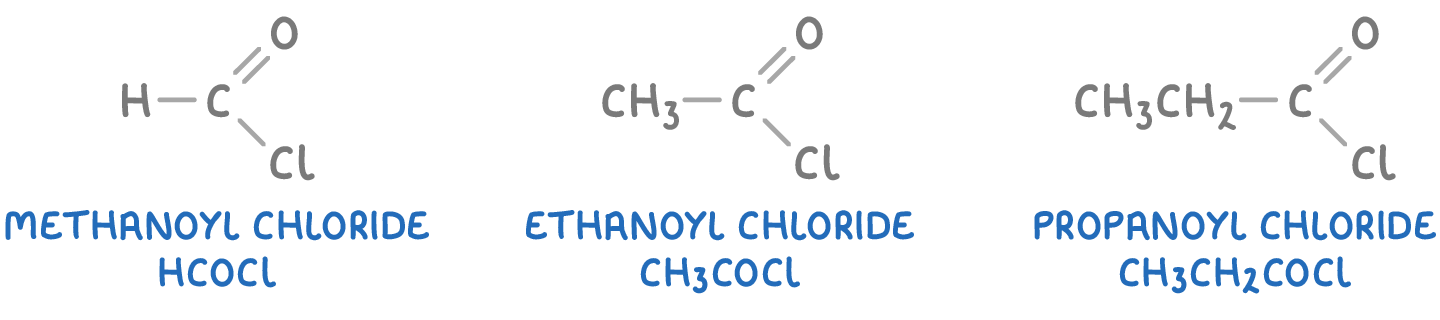
When numbering the carbon chain in an acyl chloride, the numbering starts from the end closest to the -COCl group, similar to carboxylic acids.
Acyl chlorides are made from carboxylic acids
Acyl chlorides can be synthesised from carboxylic acids using several reagents.
1. With phosphorus(V) chloride (PCl5)
- Carried out at room temperature
- For example, ethanoic acid reacts with PCl5 according to the equation: CH3COOH + PCl5 ➔ CH3COCl + POCl3 + HCl
2. With phosphorus(III) chloride (PCl3)
- Requires heating
- For example, ethanoic acid reacts with PCl3 according to the equation: 3CH3COOH + PCl3 ➔ 3CH3COCl + H3PO3
3. With thionyl chloride (SOCl2)
- Carried out at room temperature
- For example, ethanoic acid reacts with SOCl2 according to the equation: CH3COOH + SOCl2 ➔ CH3COCl + SO2 + HCl
These reactions replace the -OH group in carboxylic acids with a -Cl atom, forming acyl chlorides. Thionyl chloride is especially useful for this reaction because the by-products, SO2 and HCl, are gaseous and escape the reaction mixture, driving the reaction towards completion.
Reactions of acyl chlorides
Acyl chlorides are highly reactive due to their polar C=O bond and the chlorine atom, which is readily displaced.
They can react with:
- Water - This reaction is vigorous, even at low temperatures, and reforms the carboxylic acid. The reaction also produces steamy fumes of hydrogen chloride gas. For example, ethanoyl chloride is hydrolysed to ethanoic acid according to the equation:
CH3COCl + H2O ➔ CH3COOH + HCl
- Alcohols - At room temperature, this reaction is vigorous, producing an ester and HCl. For example, ethanoyl chloride reacts with ethanol to form ethyl ethanoate according to the equation:
CH3COCl + C2H5OH ➔ CH3COOC2H5 + HCl
- Ammonia - This reaction occurs violently at room temperature, resulting in a primary amide and NH4Cl. For example, ethanoyl chloride reacts with ammonia to form ethanamide according to the equation:
CH3COCl + 2NH3 ➔ CH3CONH2 + NH4Cl
- Primary amines - This reaction occurs violently at room temperature, resulting in a secondary amide and (CH3)2NH2+Cl-. For example, ethanoyl chloride reacts with methylamine to form N-methylethanamide according to the equation:
CH3COCl + 2CH3NH2 ➔ CH3CONHC2H5 + (CH3)2NH2Cl
- Phenol - Though slower, this reaction at room temperature is useful for forming phenolic esters, esterifying the typically unreactive phenol. For example, ethanoyl chloride reacts with phenol to form phenyl ethanoate according to the equation:
CH3COCl + C6H5OH ➔ CH3COOC6H5 + HCl
Acyl chlorides react via addition-elimination
The reaction mechanism for acyl chlorides involves nucleophilic addition-elimination:
- Addition - A nucleophile attacks the carbonyl carbon, which carries a partial positive charge, leading to the formation of a tetrahedral intermediate.
- Elimination - The chloride ion leaves, and the carbonyl group is reformed, with HCl being released.
For example, the mechanism using water as the nucleophile is shown below (R = alkyl group):
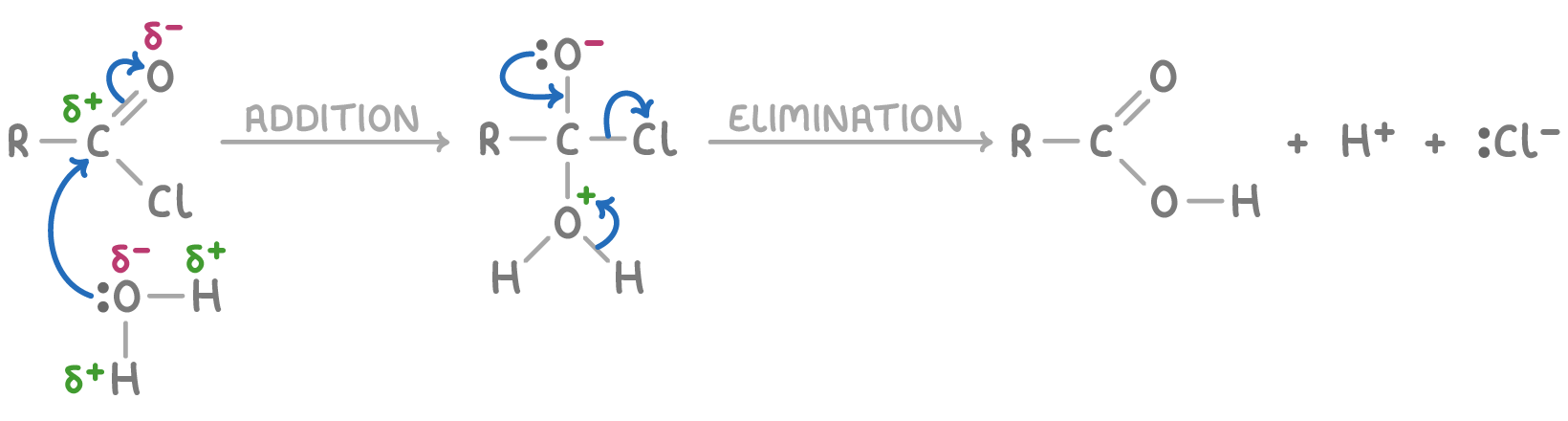
Relative ease of hydrolysis
The ease with which acyl chlorides, alkyl chlorides and aryl chlorides undergo hydrolysis follows this order:
acyl chlorides > alkyl chlorides > aryl chlorides
Acyl chlorides readily hydrolyse at room temperature with a neutral water molecule acting as the nucleophile:
RCOCl + H2O ➔ RCOOH + HCl
Alkyl chlorides require heating under reflux with aqueous sodium hydroxide acting as the strong nucleophilic hydroxide ion (OH-) to facilitate hydrolysis:
RCl + NaOH ➔ ROH + NaCl
Reasons for differences in hydrolysis rate
Aryl chlorides such as chlorobenzene do not undergo hydrolysis under typical conditions.
Acyl chlorides hydrolyse rapidly due to:
- The strongly electron-withdrawing carbonyl group adjacent to the carbon-chlorine bond.
- This makes the carbon highly positive and susceptible to nucleophilic attack by water.
Alkyl chlorides hydrolyse slower than acyl chlorides because:
- The carbon bonded to the chlorine does not carry a significant partial positive charge.
- A stronger nucleophile (OH-) and heat are required to initiate hydrolysis.
Aryl chlorides do not undergo hydrolysis because:
- The chlorine atom's p-orbital overlaps with the aromatic ring's π-system.
- This makes the carbon-chlorine bond particularly strong, preventing nucleophilic attack by water.