Similarities and Trends in Properties of Group 2 Compounds
This lesson covers:
- Thermal stability of group 2 carbonates and nitrates
- Solubility trends for group 2 hydroxides and sulfates
Thermal stability depends on degree of anion polarisation
Group 2 carbonates decompose on heating to metal oxides and carbon dioxide, for example:
CaCO3(s) ➔ CaO(s) + CO2(g)
Group 2 nitrates decompose on heating to metal oxides, nitrogen dioxide and oxygen, for example:
2Mg(NO3)2(s) ➔ 2MgO(s) + 4NO2(g) + O2(g)
The trend in thermal stability of these carbonates and nitrates can be explained using the concepts of ion polarisation and polarising power.
Ion polarisation is the distortion of the electron cloud around an anion caused by the electric field of a nearby cation attracting the electrons. The polarising power of a cation refers to its ability to polarise nearby anions.
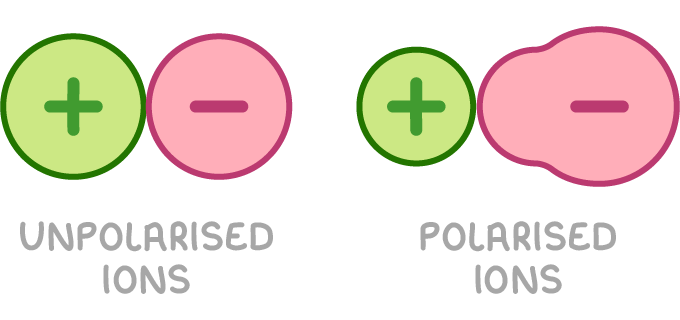
The thermal stability of group 2 carbonates and nitrates increases down the group because:
- The ionic radius of the group 2 cation increases down the group.
- As ionic radius increases, the charge density decreases.
- Cations with a lower charge density have a lower polarising power and are less able to polarise the large carbonate and nitrate anions.
- Less polarisation leads to less distortion of the anion's electron cloud and stronger C-O and N-O bonds.
- More energy is needed to break these bonds in the anion to form carbon dioxide and nitrogen dioxide.
So the carbonates and nitrates of magnesium have the lowest thermal stability and require the lowest temperatures to decompose, whereas barium carbonate and barium nitrate have the highest thermal stability and require the highest temperatures to decompose.
Solubility depends on relative lattice energies and hydration enthalpies
The solubility of group 2 hydroxides and sulfates depend on their enthalpy change of solution (ΔHsol).
This can be related to solubility using Hess's law:
ΔHsol=ΔHhyd−ΔHlatt
The more positive (or less negative) their ΔHsol value, the lower their solubility.
Therefore, solubility depends on the relative magnitudes of:
- Enthalpy change of hydration
- Lattice energy
Trend in solubility of group 2 hydroxides
The solubility of group 2 hydroxides increases down the group because:
- The charge density of the group 2 cation decreases down the group.
- This causes both the enthalpy change of hydration and the lattice energy to become less negative.
- The small hydroxide ion makes a relatively small contribution to the lattice energy so lattice energy is determined more by the larger group 2 cations.
- Lattice energy changes by a greater amount compared to the enthalpy change of hydration.
- ΔHsol becomes more negative (or less positive) down the group.
Trend in solubility of group 2 sulfates
The solubility of group 2 sulfates decreases down the group because:
- The charge density of the group 2 cation decreases down the group.
- This causes both the enthalpy change of hydration and the lattice energy to becomes less negative.
- The large sulfate ion makes a relatively large contribution to the lattice energy so lattice energy is determined more by the larger sulfate anion.
- Enthalpy change of hydration changes by a greater amount compared to the lattice energy.
- ΔHsol becomes less negative (or more positive) down the group.