Reactions of Aldehydes & Ketones
This lesson covers:
- Oxidation of aldehydes to carboxylic acids
- Reduction of aldehydes and ketones to alcohols
- Hydroxynitrile formation
Aldehydes are easily oxidised to carboxylic acids
Aldehydes can be oxidised by oxidising agents such as acidified potassium dichromate(VI) to form carboxylic acids. The colour change observed during this oxidation is from orange to green, as the Cr(VI) in the dichromate ion is reduced to Cr(III). However, ketones do not undergo oxidation under the same conditions, so colour change is seen.
For example, methanal is oxidised to methanoic acid:
HCHO + [O] ➔ HCOOH
By contrast, propanone does not react with oxidising agents:
CH3COCH3 + [O] ➔ no reaction
This difference in reactivity occurs because the hydrogen atom attached to the carbonyl carbon in an aldehyde is weakly acidic, so it is easily displaced. By contrast, ketones lack that weakly acidic hydrogen, so oxidation does not occur.
Aldehydes and ketones form hydroxynitriles
Aldehydes and ketones can react with hydrogen cyanide (HCN) in nucleophilic addition reactions to produce hydroxynitriles which conains both the cyano (-CN) and hydroxy (-OH) substituents. This reaction is useful because it increases the length of the carbon chain by 1 carbon atom.
The reactions are:
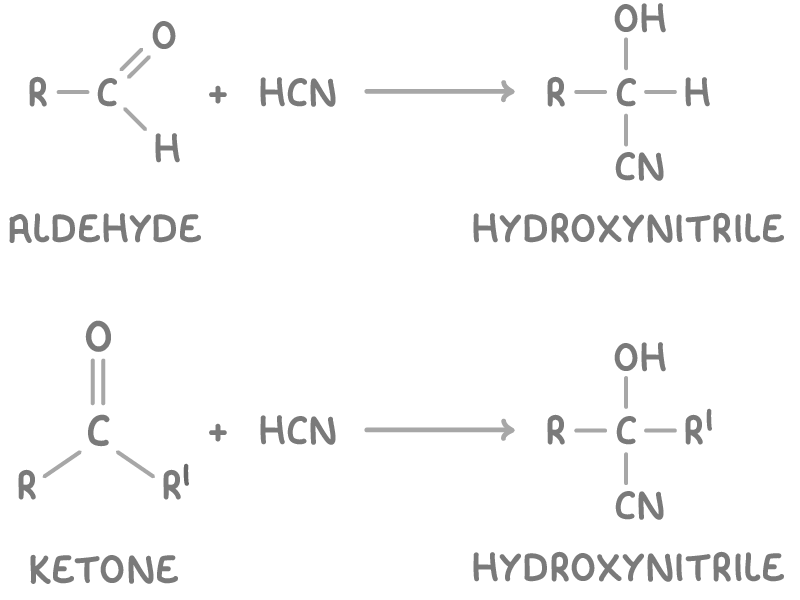
Where
- R and R’ represent alkyl groups.
Hydrogen cyanide is toxic so it is often generated in situ by mixing sodium cyanide or potassium cyanide with sulfuric acid.
Mechanism of hydroxynitrile formation
The formation of hydroxynitriles from carbonyl compounds, such as ethanal, follows a nucleophilic addition mechanism:
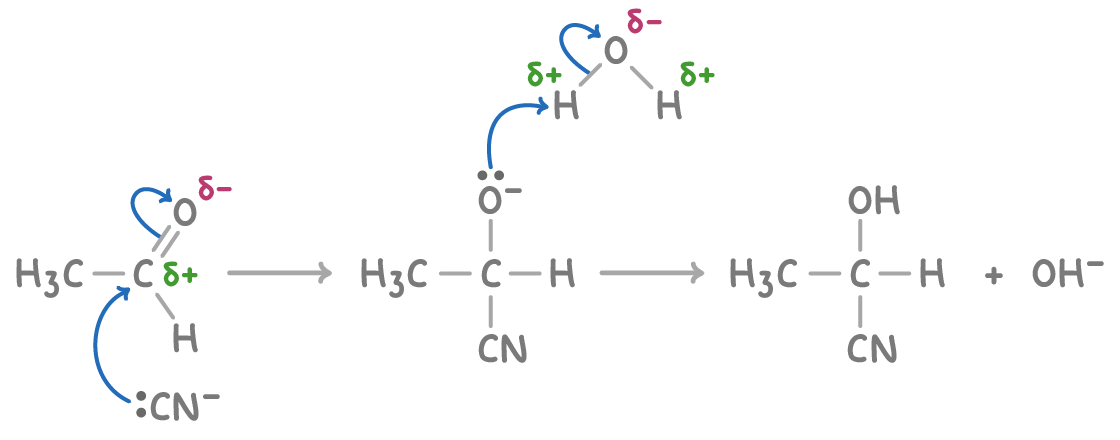
- CN- attacks the partially positive carbonyl carbon, transferring the electrons to oxygen.
- Protonation of oxygen by water (or acid) to give the -OH group.