Making Halogenoalkanes
This lesson covers:
- How to name halogenoalkanes
- How halogenoalkanes are classified as primary, secondary or tertiary
- Reactions to produce halogenoalkanes
- Polarity of the carbon-halogen bond
- Trend in hydrolysis rates of halogenoalkanes
- Comparing halogenoalkane reactivity experimentally
Naming halogenoalkanes
A halogenoalkane is a type of chemical compound where one or more hydrogen atoms in an alkane have been replaced by halogen atoms (like fluorine, chlorine, bromine, or iodine).
To name a halogenoalkane, we use prefixes (like fluoro-, chloro-, bromo-, iodo-) to indicate the type and number of halogen atoms.
Here are some examples of halogenoalkanes:
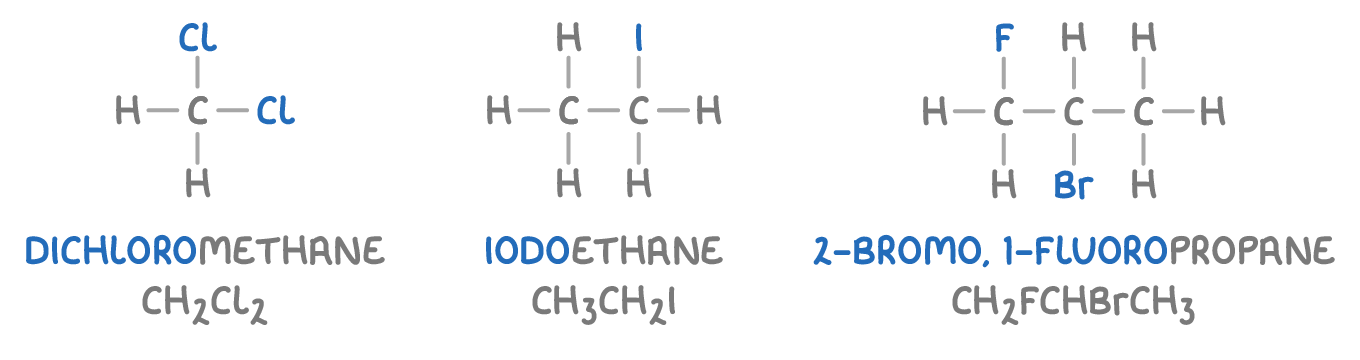
Types of halogenoalkanes
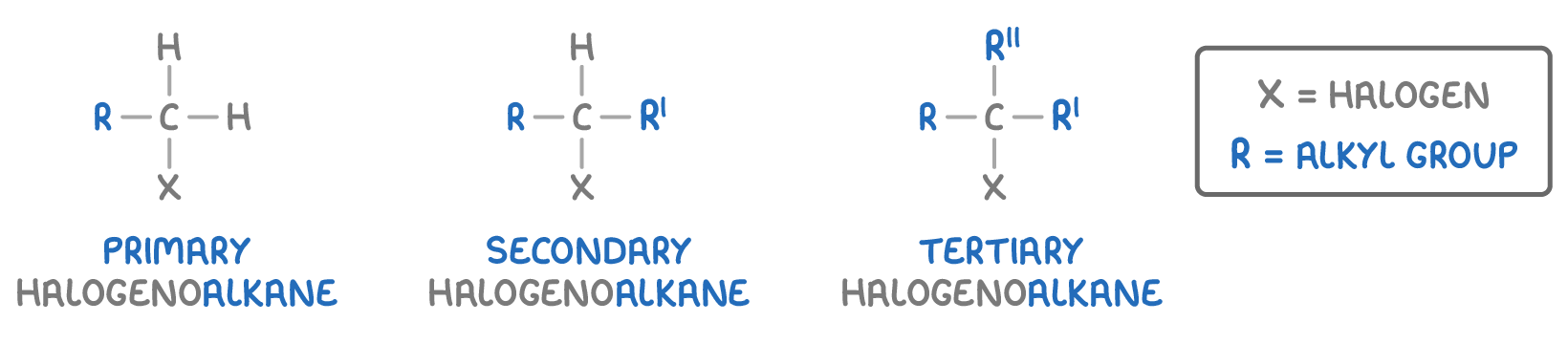
Halogenoalkanes with one substituted halogen atom can be categorised based on the groups attached to the carbon with the halogen:
- Primary - The halogen is attached to a carbon atom connected to only one alkyl group (or no alkyl groups).
- Secondary - The halogen is attached to a carbon atom connected to two alkyl groups.
- Tertiary - The halogen is attached to a carbon atom connected to three alkyl groups.
Reactions to produce halogenoalkanes
Halogenoalkanes can be produced via several reactions:
- Free-radical substitution of alkanes by Cl2 or Br2 in the presence of UV light. For example, ethane reacts with chlorine under UV light to form chloroethane:
CH3CH3 + Cl2 ➔ CH3CH2Cl + HCl
- Electrophilic addition of an alkene with a halogen or hydrogen halide at room temperature. For example, ethene reacts with bromine to form 1,2-dibromoethane:
CH2=CH2 + Br2 ➔ CH2Br-CH2Br
- Substitution of an alcohol by reaction with:
- a hydrogen halide,
- KCl and concentrated H2SO4 or concentrated H3PO4,
- PCl3 with heat,
- PCl5, or
- SOCl2.
For example, ethanol reacts with HBr to form bromoethane:
CH3CH2OH + HBr ➔ CH3CH2Br + H2O
Polarity of the carbon-halogen bond
In halogenoalkanes, the carbon-halogen bond is polar because halogen atoms have a higher electronegativity than carbon. This causes an uneven distribution of electrons, making the carbon atom partially positively charged (δ+) and the halogen atom partially negatively charged (δ-).
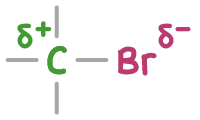
This polarity in the bond makes the carbon atom a target for nucleophiles (electron pair donors). Common nucleophiles include OH-, CN-, NH3, and H2O.
Trends in hydrolysis rates
Hydrolysis of a halogenoalkane is a reaction where the carbon-halogen bond breaks in the presence of water, forming an alcohol and a hydrogen halide.
For example:
RCl + H2O ➔ ROH + H+ + Cl-
The rate of hydrolysis depends on the bond enthalpy of the carbon-halogen bond. Bond enthalpy measures bond strength quantitatively - it is the energy required to break one mole of bonds between two atoms in the gaseous state.
Carbon-halogen bonds with lower bond enthalpies are weaker and require less energy to break, allowing them to react at a faster rate.
Carbon-halogen bond enthalpy decreases down group 7 because:
- The atomic radius of the halogen increases.
- The carbon-halogen bond length increases.
- The electrostatic attraction between bonding electrons and nuclei decreases.
- The amount of energy needed to break these longer, weaker bonds decreases.
Therefore, iodoalkanes (with the weakest carbon-halogen bonds) hydrolyse the fastest, while fluoroalkanes (with the strongest bonds) hydrolyse the slowest.
| Bond | Bond enthalpy (kJ mol-1) |
|---|---|
| C–F | 467 |
| C–Cl | 346 |
| C–Br | 290 |
| C–I | 228 |
Comparing halogenoalkane reactivity using experiments
To compare the relative reactivity of chloro-, bromo- and iodo-alkanes, a common experiment is performed:
- Place a chloroalkane, a bromoalkane, and an iodoalkane in separate test tubes.
- Add ethanol to each tube and warm them in a water bath at 50°C.
- Add silver nitrate solution to each tube. The water in the solution hydrolyses the halogenoalkane (RX):
RX(aq) + H2O(l) ➔ ROH(aq) + H+(aq) + X-(aq)
- The halide ions (X-) produced then react with the silver ions to form a silver halide precipitate:
X-(aq) + Ag+(aq) ➔ AgX(s)
- Time how long it takes for a visible precipitate to form after adding silver nitrate.
A quicker formation of precipitate indicates a faster hydrolysis. Generally, iodoalkanes show the fastest precipitate formation, and chloroalkanes the slowest.
The colour of the precipitate also helps to identify the original halogenoalkane:
- Silver chloride (AgCl) forms a white precipitate.
- Silver bromide (AgBr) forms a cream precipitate.
- Silver iodide (AgI) forms a yellow precipitate.