Producing acyl chlorides from carboxylic acids
Acyl chlorides can be prepared by reacting carboxylic acids with several chlorinating agents. The following examples feature ethanoic acid, but the reactions are applicable to other carboxylic acids as well.
- Phosphorus(III) chloride (PCl3)
Carboxylic acids react with PCl3 when heated to yield acyl chlorides. For example:
3CH3COOH + PCl3 ➔ 3CH3COCl + H3PO3
- Phosphorus(V) chloride (PCl5)
At room temperature, carboxylic acids react with PCl5 to produce acyl chlorides. For example:
CH3COOH + PCl5 ➔ CH3COCl + POCl3 + HCl
- Thionyl chloride (SOCl2)
SOCl2 reacts with carboxylic acids at room temperature, forming acyl chlorides. For example:
CH3COOH + SOCl2 ➔ CH3COCl + SO2 + HCl
In these reactions, the -OH group of the carboxylic acid is replaced by a -Cl atom, yielding the acyl chloride. The by-products (H3PO3, POCl3, HCl, SO2) are easily separated from the desired acyl chloride product.
Oxidation reactions of carboxylic acids
Under normal conditions, most carboxylic acids do not undergo further oxidation.
However, there are exceptions:
- Methanoic acid (HCOOH) is susceptible to oxidation by mild oxidising agents, such as Fehling’s solution, Tollens’ reagent, acidified K2Cr2O7 or acidified KMnO4 yielding carbon dioxide and water:
HCOOH + [O] ➔ CO2 + H2O
- Ethanedioic acid (HOOCCOOH) can be oxidised by stronger oxidising agents, such as warm acidified potassium manganate(VII) (KMnO4), to also produce carbon dioxide and water:
HOOCCOOH + [O] ➔ 2CO2 + H2O
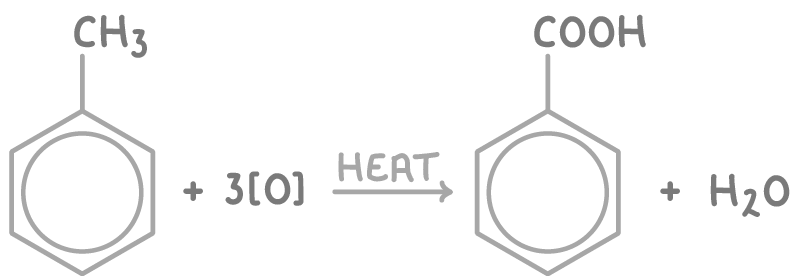
Producing benzoic acid by side chain oxidation
Benzoic acid can be produced by oxidising the methyl group of methylbenzene (an alkylbenzene). The benzene ring activates the alkyl side chain, allowing it to be oxidised.
The reaction involves heating methylbenzene with alkaline potassium manganate(VII) solution (KMnO4). This oxidises the methyl group to a carboxylate ion (-COO-). Subsequent addition of a dilute acid protonates the carboxylate, yielding benzoic acid.
The equation for this reaction is:
Electron-withdrawing groups increase acidity
Incorporating electron-withdrawing groups, such as chlorine, on the carbon atom adjacent to the carboxyl group leads to an increase in the acidity of carboxylic acids:
- These groups pull electrons away from the O–H bond, weakening it and encouraging the release of H+ ions.
- They also stabilise the negative charge on the carboxylate ion, making the dissociation process more favourable.
The impact of substituting hydrogen atoms with chlorine atoms on acidity is illustrated in the table below:
| Acid | Ka at 25°C (mol dm-3) |
|---|---|
| Ethanoic acid | 1.7 x 10-5 |
| Chloroethanoic acid | 1.3 x 10-3 |
| Dichloroethanoic acid | 5.0 x 10-2 |
| Trichloroethanoic acid | 2.3 x 10-1 |
The addition of highly electronegative chlorine atoms reduces the Ka value and enhances the acid's strength.
Trichloroethanoic acid is the strongest acid due to the significant electron-withdrawing effect of its three chlorine atoms.
Carboxylic acids are more acidic than alcohols and phenols
Carboxylic acids are found to be stronger acids than both alcohols and phenols.
This difference in acid strength is due to two main factors:
1. The C=O double bond draws electron density away from the O–H bond, making it weaker and more prone to dissociation.
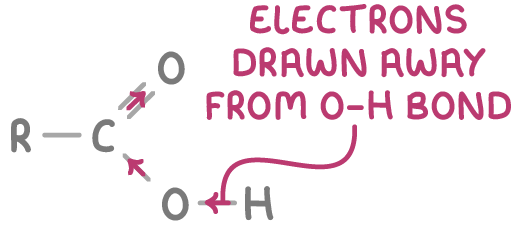
2. Delocalisation of electrons within the carboxylate ion stabilises the negative charge, further facilitating dissociation.
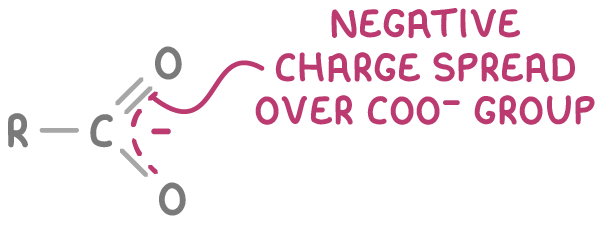
Phenols exhibit greater acidity than alcohols but are still less acidic than carboxylic acids.
The following table compares their acid strengths:
| Compound | Ka at 25°C (mol dm-3) |
|---|---|
| Ethanoic acid | 1.7 x 10-5 |
| Phenol | 1.0 x 10-10 |
| Ethanol | 1 x 10-16 |
Carboxylic acids as weak acids
Carboxylic acids, such as ethanoic acid (CH3COOH), exhibit typical acid behaviour when dissolved in water:
- They dissociate partially to release H+(aq) ions:
- CH3COOH(aq) ⇌ CH3COO-(aq) + H+(aq)
- They react with bases, like NaOH, to form salts (known as carboxylates) and water:
- CH3COOH(aq) + NaOH(aq) ➔ CH3COO- Na+(aq) + H2O(l)
However, carboxylic acids are considered weak acids because the equilibrium of the dissociation reaction lies significantly to the left, indicating that most of the acid molecules remain undissociated:
Ka (ethanoic acid) = 1.7 x 10-5 mol dm-3 at 25 °C
The lower the Ka value, the weaker the acid.
Carboxylic Acids
This lesson covers:
- Carboxylic acids as weak acids
- Comparison of acid strength between carboxylic acids, phenols, and alcohols
- Effect of electronegative substituents on carboxylic acid strength
- Synthesis of benzoic acid
- Oxidation reactions of carboxylic acids
- Synthesis of acyl chlorides