Halogen Compounds
This lesson covers:
- Electrophilic substitution reactions of arenes with halogens
- The mechanism of electrophilic substitution
- Activating groups and preferred substitution positions
- Side chain halogenation of alkylbenzenes
- Comparing reactivity of chlorobenzene and chloroethane
Electrophilic substitution maintains aromaticity
Benzene and other arenes readily undergo electrophilic substitution reactions with halogens. This involves a halogen atom replacing a hydrogen atom on the aromatic ring.
Importantly, these substitution reactions occur without disrupting the aromatic stability provided by the ring of delocalised pi electrons. Opening up the ring to form an addition product would destroy this stability.
Halogenation using a halogen carrier
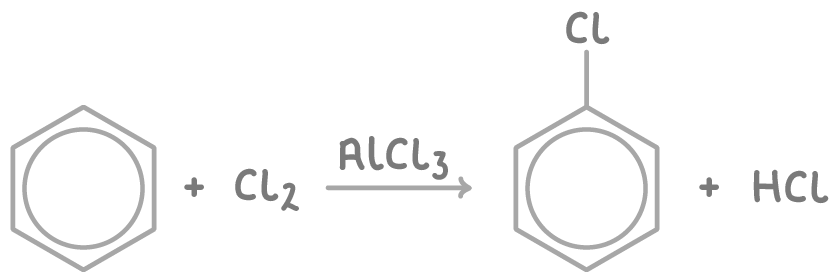
Benzene reacts with chlorine when an anhydrous aluminium chloride catalyst is present.
The reaction is:
The electrophile is created when the AlCl3 catalyst polarises the Cl-Cl bond:
- A chlorine atom forms a coorinate bond to the catalyst, donating electrons.
- This pulls electrons away from the other chlorine, making it partially positive (Cl+):
Cl2 + AlCl3 ➔ Cl+ + AlCl4-
- The resultant Cl+ electrophile attacks the electron-rich benzene ring to form chlorobenzene and displacing H+.
- The H+ and AlCl4- combine to regenerate the catalyst:
H+ + AlCl4- ➔ AlCl3 + HCl
Catalysts such as AlCl3 that carry and activate the halogen are called halogen carriers.
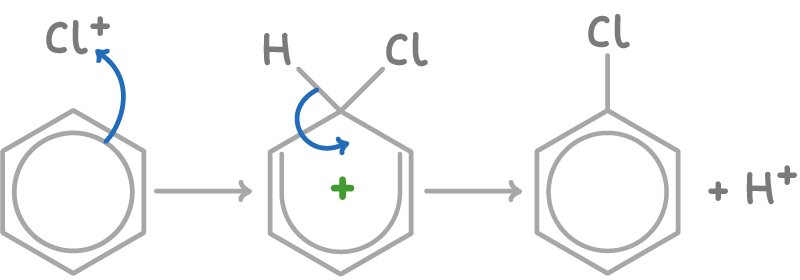
Mechanism of electrophilic aromatic substitution
The reaction mechanism involves two steps:
- The electrophile attacks the π electron cloud, disrupting aromaticity. A carbocation intermediate is formed.
- A proton is lost from the intermediate, reforming the aromatic ring with the substituent now attached.
For example, the mechanism to form chlorobenzene is:
The formation of bromobenzene occurs via similar electrophilic substitution mechanism using a Br+ electrophile and a AlBr3 catalyst.
Activating groups determine substitution position
With substituted arenes like alkylbenzenes, the halogen enters the ring at the 2- and 4- positions from the activating groups already attached. These positions are activated by the electron-donating effects of the substituents.
Other activating groups that direct substitution to these positions include -OH and -NH2.
For example, chlorinating methylbenzene using a halogen carrier catalyst produces a mixture of 2-chloromethylbenzene and 4-chloromethylbenzene.
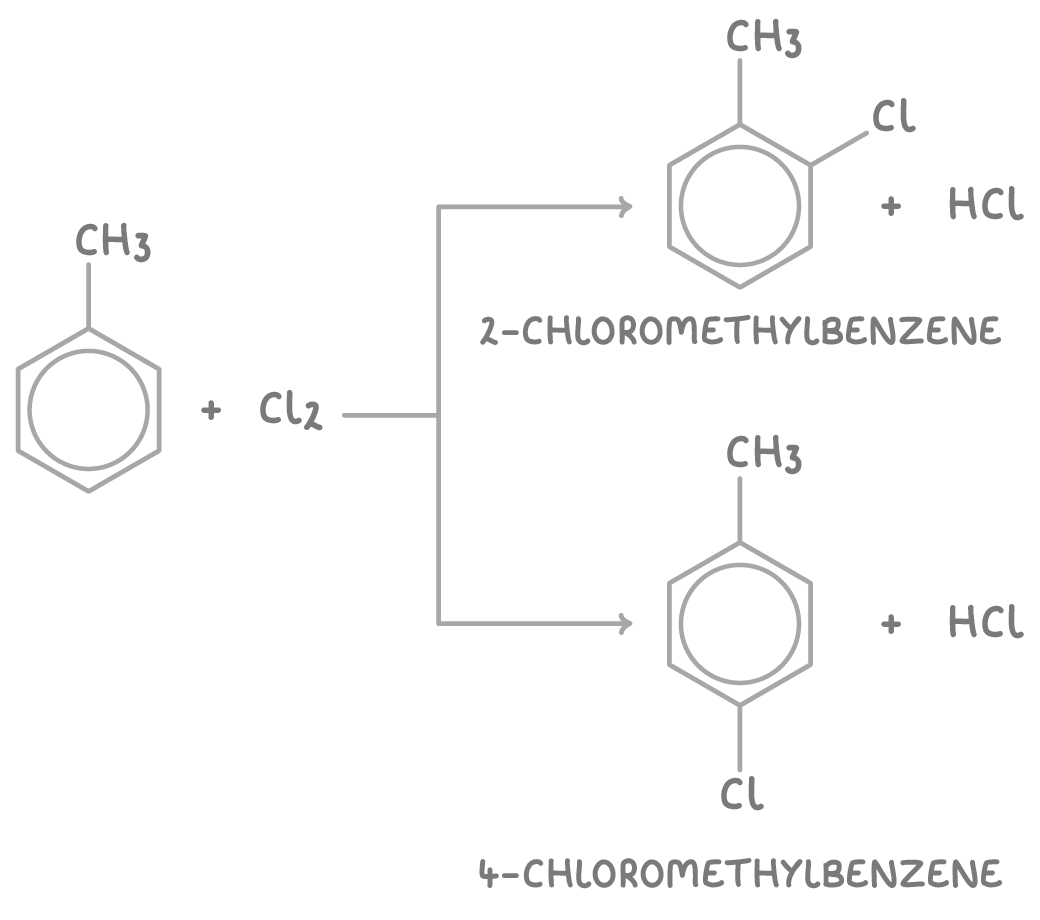
With excess chlorine, multi-substitution can occur to give di- and tri-chloro products.
Side chain reactions under different conditions
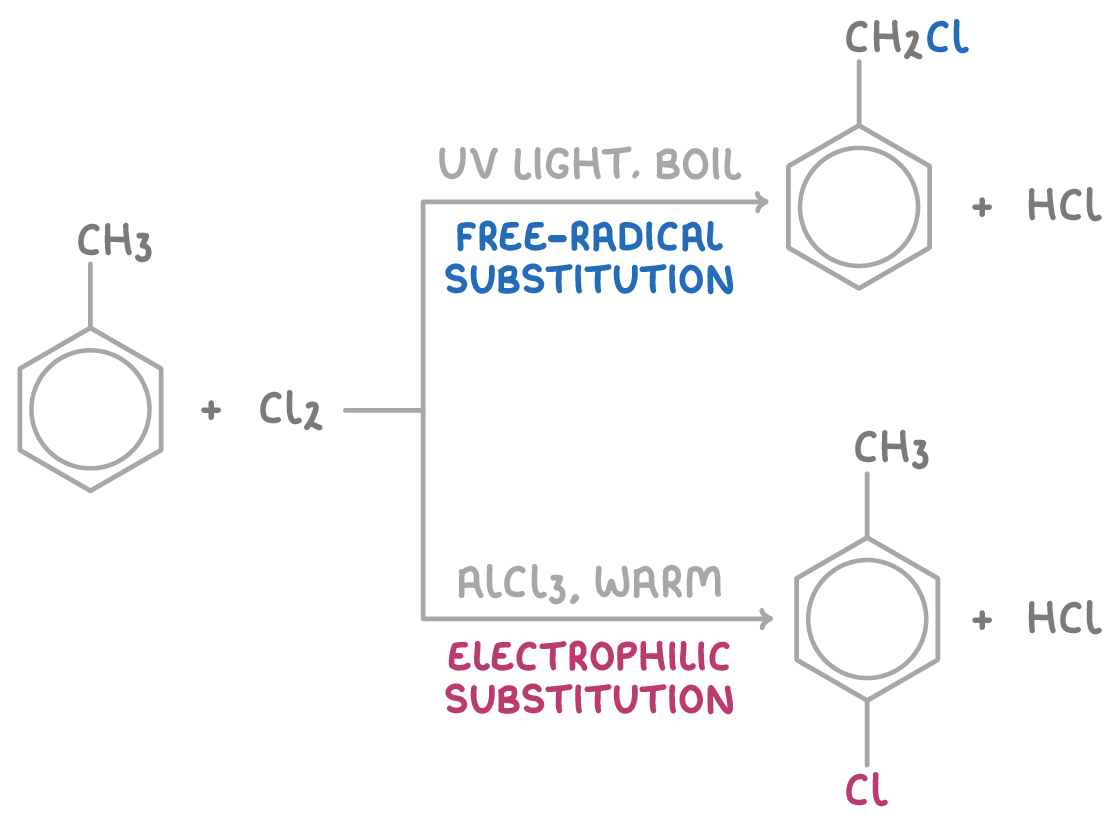
Under electrophilic substitution conditions, only ring substitution occurs, not side chain halogenation.
But side chains can undergo free radical halogenation with chlorine if:
- Ultraviolet (UV) light is present, and
- The reaction mixture is boiled
This leads to side chain substitution, without ring halogenation.
For example, the reaction conditions dictate whether methylbenzene undergoes free radical substitution or electrophilic substitution with chlorine.
Comparing chlorobenzene and chloroethane reactivity
Halogenoarenes like chlorobenzene (C6H5Cl) are far less reactive than halogenoalkanes like chloroethane (C2H5Cl).
The decreased reactivity of halogenoarenes like chlorobenzene occurs due to effects of the aromatic ring:
- One of the chlorine atom's lone pairs can delocalise into the aromatic π electron system of the ring.
- Delocalisation gives the carbon-chlorine bond a partial double bond character.
- This partial double bond increases the bond strength, making it more difficult to break.