Naming Organic Compounds
This lesson covers:
- The key homologous series of organic compounds
- Naming rules and conventions for esters
- Naming aromatic compounds derived from benzene
Homologous series of organic compounds
Organic compounds can be categorised into homologous series based on similarities in their molecular structures.
Some of the main homologous series are listed below, along with the prefixes, suffixes and functional groups used to identify them:
| Homologous series | Functional group | Suffix | Example |
|---|---|---|---|
| Alkane | N/A | -ane | Ethane, C2H6 |
| Alkene | C=C | -ene | Ethene, C2H4 |
| Alcohol | O-H | -ol | Ethanol, C2H5OH |
| Aldehyde | C=O | -al | Ethanal, CH3CHO |
| Ketone | C=O | -one | Propanone, CH3COCH3 |
| Carboxylic acid | COOH | -oic acid | Ethanoic acid, CH3COOH |
| Amine | N-H | -amine | Ethylamine, CH3CH2NH2 |
| Nitrile | C≡N | -nitrile | Ethanenitrile, CH3CN |
| Amide | CON | -amide | Ethanamide, CH3CONH2 |
| Acyl chloride | COCl | -oyl chloride | Ethanoyl chloride, CH3COCl |
| Homologous series | Functional group | Prefix | Example |
|---|---|---|---|
| Branched alkanes | N/A | alkyl- | Methylpropane, (CH3)2CHCH3 |
| Cycloalkanes | N/A | cyclo- | Cyclohexane, C6H12 |
| Halogenoalkanes | C-X | fluoro-, chloro-, bromo-, iodo- | Chloroethane, CH3CH2Cl |
| Hydroxynitrile | C(OH)C≡N | hydroxy- | 2-hydroxypropanenitrile, CH3CH(OH)CN |
Naming esters
Esters are formed from reacting an alcohol with a carboxylic acid.
Esters contain the functional group C-O-C=O.
Rules for naming esters:
- Identify the alkyl group from the alcohol - this gives the first part of the ester name.
- Identify the acid group and replace the '-oic acid' ending with '-oate' - this gives the second part of the name.
- Put the two parts together to give the complete ester name.
For example, the ester below is named propyl ethanoate:
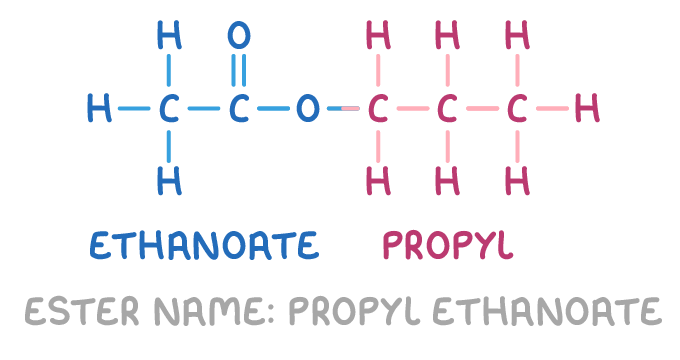
This is because:
- It contains a 3-carbon alkyl chain from the alcohol (indicated in red). This is recognised as a propyl group.
- It contains a 2-carbon chain from the acid (indicated in blue) with the ester bond -COO- in the middle. Converting the acid name ethanoic acid to an -oate ending gives ethanoate.
- Putting the two parts together, the alcohol-derived propyl group at the front and the acid-derived ethanoate group at the end, gives the complete name propyl ethanoate.
Naming aromatic compounds
Aromatic compounds containing benzene rings are called arenes.
Rules for naming arenes:
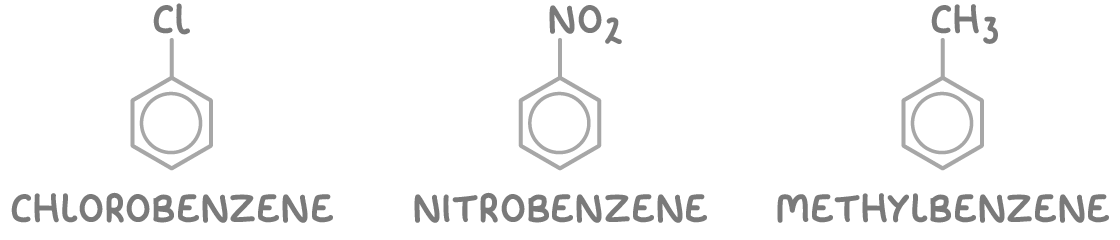
1. Some arenes are named as substituted benzene derivatives using the suffix -benzene.
These include:
- Halogenoarenes (e.g. chlorobenzene)
- Nitroarenes (e.g. nitrobenzene)
- Alkylarenes (e.g. methylbenzene)
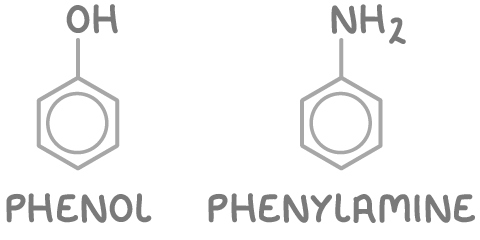
2. Others arenes are named as phenyl (C6H5) derivatives using prefix phenyl-.
These include:
- Phenol
- Phenylamine
3. Arenes with multiple substituents are named by numbering the carbon atoms in the benzene ring such that the substituents receive the lowest possible numbers. The numbering begins at the carbon atom with the functional group that determines the suffix of the compound's name.
For example, the arene below is named 2,4-dibromophenylamine:
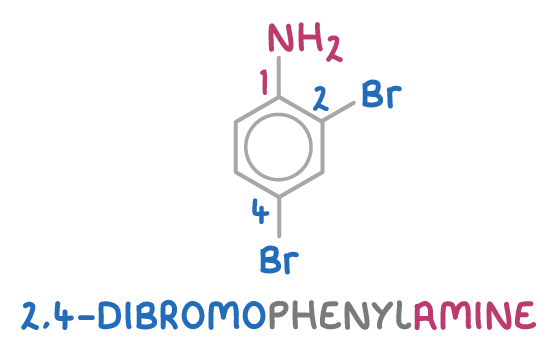
This is because:
- It contains an -NH2 functional group, which means the compound is an amine. Therefore, the suffix should be -phenylamine based on the presence of the amino group.
- Numbering from the amino group gives the bromine substituents positions 2 and 4. Therefore, the full name of this compound is 2,4-dibromophenylamine.