Catalysis
This lesson covers:
- The difference between homogeneous and heterogeneous catalysis
- The mechanism of homogeneous catalysis with examples
- The mechanism of heterogeneous catalysis with examples
Catalysts and types of catalysis
Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process. They achieve this by providing an alternative reaction pathway with a lower activation energy (Ea).
Catalysis can be classified into two main types based on the phase of the catalyst relative to the reaction mixture:
- Homogeneous catalysis - The catalyst and the reactants are in the same phase, usually liquid.
- Heterogeneous catalysis - The catalyst and the reactants are in different phases, typically a solid catalyst with liquid or gaseous reactants.
Mechanism of homogeneous catalysis
Homogeneous catalysis often involves transition metal ions undergoing redox cycles, where the metal ion's oxidation state changes during the reaction. The catalyst participates in one step and is regenerated in a later step, allowing it to be reused in the catalytic cycle.
The general mechanism can be summarised as:
- The catalyst (often a transition metal ion) reacts with a reactant, changing its oxidation state and forming a catalyst-reactant intermediate.
- The intermediate then reacts with another reactant to form the product and regenerate the original catalyst.
Examples of homogeneous catalysis
1. Fe3+ ions in the reaction between S2O82- and I-
The redox reaction between iodide ions (I-) and peroxodisulfate ions (S2O82-) is catalysed by Fe3+ ions:
S2O82-(aq) + 2I-(aq) ➔ 2SO42-(aq) + I2(aq)
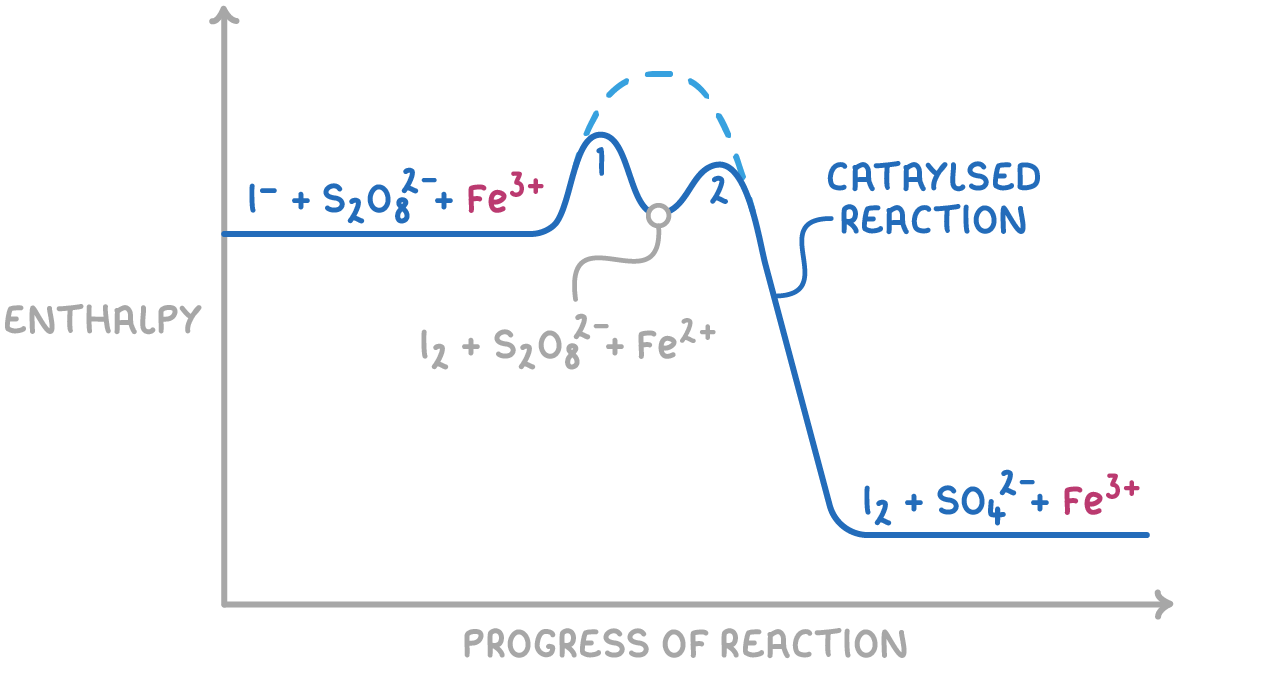
The mechanism involves two redox steps:
- Reduction of Fe3+ to Fe2+ by I-:
2Fe3+(aq) + 2I-(aq) ➔ 2Fe2+(aq) + I2(aq)
- Oxidation of Fe2+ back to Fe3+ by S2O82-:
2Fe2+(aq) + S2O82-(aq) ➔ 2Fe3+(aq) + 2SO42-(aq)
Fe2+ can also catalyse this reaction, with the redox steps occurring in the reverse order.
2. NO2 in the oxidation of sulfur dioxide
Nitrogen dioxide (NO2) catalyses the oxidation of sulfur dioxide (SO2) to sulfur trioxide (SO3) in the atmosphere, contributing to acid rain formation:
SO2(g) + NO2(g) ➔ SO3(g) + NO(g)
The NO2 catalyst is regenerated by the oxidation of NO by atmospheric O2:
2NO(g) + O2(g) ➔ 2NO2(g)
Mechanism of heterogeneous catalysis
Heterogeneous catalysis involves the adsorption of reactants onto the surface of a solid catalyst, where they react to form products that then desorb from the surface. The catalyst provides a surface for the reaction to occur on, and can also participate in the reaction by forming temporary bonds with the reactants.
The mechanism of heterogeneous catalysis for the synthesis of ammonia using an iron catalyst is shown below.
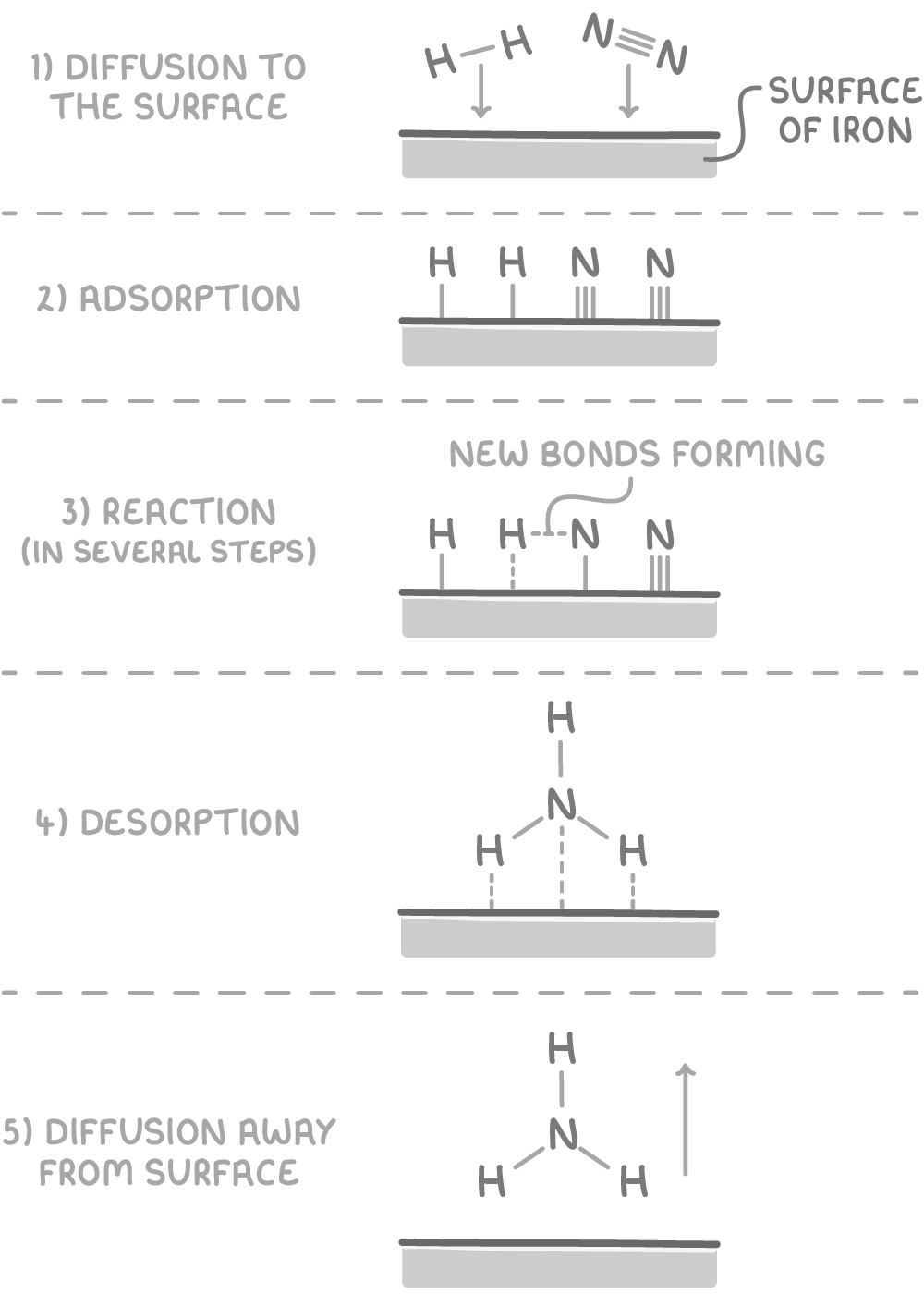
The key steps in the mechanism are:
- Diffusion of reactants to the catalyst surface.
- Adsorption of reactants onto the surface, forming bonds with surface atoms and weakening bonds within the reactant molecules.
- Formation of new bonds through the reaction of the adsorbed reactant species, leading to the formation of products.
- Desorption of products from the surface.
- Diffusion of products away from the catalyst.
Examples of heterogeneous catalysis
1. Iron in the Haber process
- Iron catalyses the formation of ammonia from nitrogen and hydrogen:
N2(g) + 3H2(g) ➔ 2NH3(g)
2. Platinum, palladium and rhodium in catalytic converters
- Platinum, palladium and rhodium are used in catalytic converters to reduce harmful exhaust emissions from vehicles.
- These transition metals catalyse chemical reactions that convert harmful exhaust gases into less harmful gases before they are released into the atmosphere.
- For example, they catalyse the conversion of nitrogen oxides and carbon monoxide into nitrogen and carbon dioxide:
NO(g) + CO(g) ➔ 1⁄2N2(g) + CO2(g)
NO2(g) + 2CO(g) ➔ 1⁄2N2(g) + 2CO2(g)