The shape of organic molecules
The shapes and bonding patterns of organic molecules can be explained by considering the arrangement of σ and π bonds.
Ethane (H3C-CH3):
Ethane consists entirely of σ (single) bonds formed by the linear overlap of hybridised sp³ orbitals. The electron density is evenly distributed, resulting in a tetrahedral geometry with H-C-H bond angles of approximately 109.5°.
Ethene (H2C=CH2):
The carbon atoms in ethene form a double bond. Each carbon atom forms three σ bonds with two hydrogen atoms and one carbon atom using sp2 hybridised orbitals. The molecule has a trigonal planar geometry with bond angles of 120°.
The remaining p orbitals on each carbon atom overlap sideways, forming a π bond.
Hydrogen cyanide (H-C≡N):
In hydrogen cyanide, the carbon and nitrogen atoms form a triple bond. The σ bond forms between the carbon and hydrogen through sp hybrid orbitals, and between the carbon and nitrogen through sp orbital overlap.
The remaining p orbitals on carbon and nitrogen overlap sideways, forming the two π bonds oriented at right angles to each other. The molecule has a linear geometry with a bond angle of 180°.
Similarly, in the linear nitrogen molecule (N2), a triple bond is formed by the overlap of sp-hybridised orbitals creating a σ bond, and the sideways overlap of p orbitals forming two π bonds.
Pi (π) bonds
Pi bonds are formed when atomic orbitals, specifically p orbitals, overlap in a sideways manner, above and below the sigma bond.
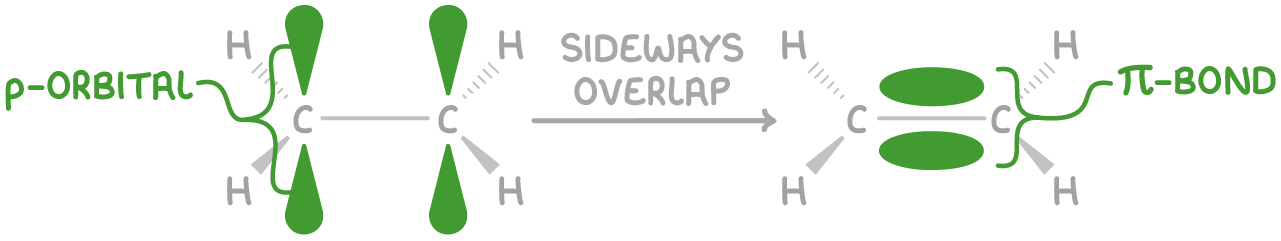
Key features of pi (π) bonds:
- The electron density is not symmetrically distributed around the internuclear axis.
- Often represented as two electron clouds, one from each p orbital lobe, but together forming a single π bond containing two electrons.
- A double bond is formed from one σ bond and one π bond.
- A triple bond is formed from one σ bond and two π bonds. The π bonds are oriented at right angles to each other.
Sigma (σ) bonds
Sigma bonds are formed when atomic orbitals overlap directly in a linear, end-on fashion.
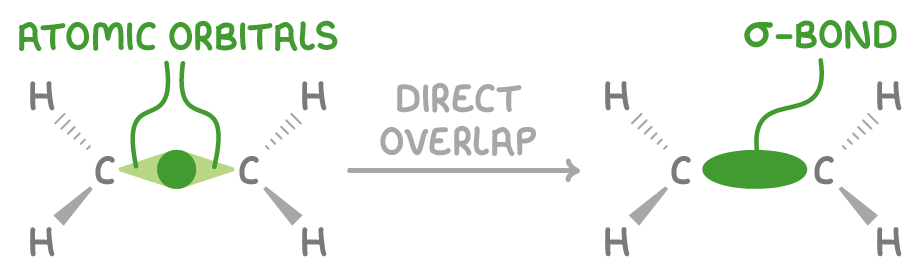
Key features of sigma (σ) bonds:
- The electron density is symmetrically distributed around the internuclear axis joining the bonded atoms.
- All single bonds in organic molecules are σ bonds.
Hybridisation of orbitals
When atomic orbitals combine to form covalent bonds, they undergo a process called hybridisation, where an s orbital mixes with one, two, or three p orbitals. The hybrid orbitals that form are more directional than the original atomic orbitals, enabling greater overlap between the atomic orbitals of bonding atoms, resulting in stronger covalent bonds and more stable molecules.
- sp³ hybridisation - One s orbital and three p orbitals mix, forming four equivalent sp³ hybrid orbitals.
- sp² hybridisation - One s orbital and two p orbitals mix, forming three equivalent sp² hybrid orbitals.
- sp hybridisation - One s orbital and one p orbital mix, forming two equivalent sp hybrid orbitals.
Covalent bond formation
Covalent bonds are formed when the atomic orbitals of two non-metal atoms overlap, allowing electrons to be shared between them. The extent of this orbital overlap determines the bond strength - greater overlap leads to stronger bonds.
For example, the 1s orbitals of two hydrogen atoms overlap to form a covalent bond.
Sigma Bonds and Pi Bonds
This lesson covers:
- The formation of covalent bonds through orbital overlap
- Hybridisation of atomic orbitals
- Sigma (σ) and pi (π) bonds and their characteristics
- The shapes and bonding patterns of ethane, ethene, and hydrogen cyanide