The Colour of Complexes
This lesson covers:
- How ligands split the energy levels of transition metal ions
- Why transition metal complexes have distinctive colours
- Factors affecting the colour of transition metal ions
Ligands split the 3d orbitals into two energy levels
When ligands bond to transition metal ions, they split the energies of the 3d orbitals into two levels:
- Normally, all the 3d orbitals have equal energy (orbitals with equal energies are termed degenerate).
- Bonding to the ligands raises the energy of some of the orbitals (creating non-degenerate orbitals).
- This splits them into a ground state (lower energy) and an excited state (higher energy).
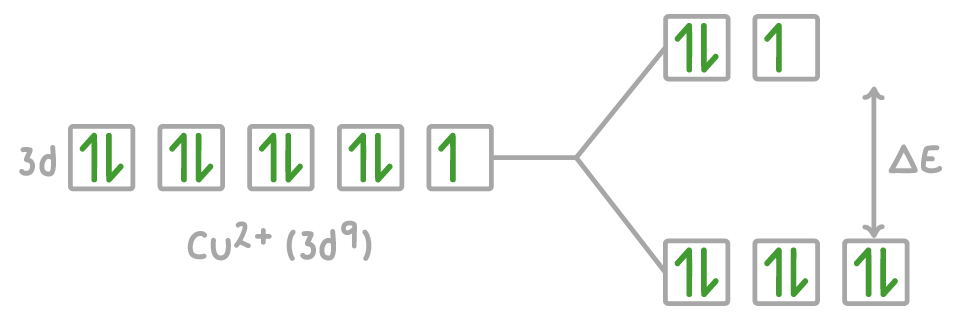
In octahedral complexes such as [Cu(H2O)6]2+, three d-orbitals have a lower energy and two d-orbitals have a higher energy.
In tetrahedral complexes such [CoCl4]2-, two d-orbitals have a lower energy and three d-orbitals have a higher energy.
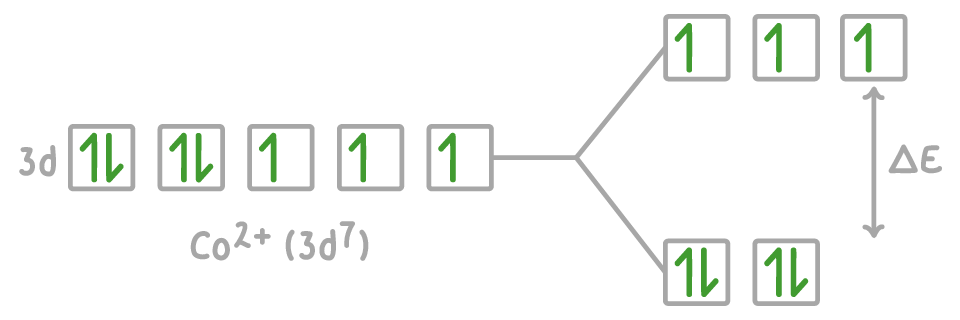
For an electron to jump from the ground state to the excited state, it needs an energy input equal to the energy gap (ΔE) between the two states.
The 4 factors that affect the energy gap (ΔE)
The energy gap, ΔE, depends on the following factors:
- Identity of the central transition metal ion
- Oxidation state of the ion
- Identity of the ligands
- Coordination number
Changing these factors will change the colour of the complex ion, for example:
- Change in central ion - e.g., [Co(H2O)6]2+ is pink whereas [Cu(H2O)6]2+ is pale blue.
- Change in oxidation state - e.g., [V(H2O)6]2+ is violet whereas [V(H2O)6]3+ is green.
- Change in ligands - e.g., [Co(H2O)6]2+ is pink whereas [Co(NH3)6]2+ is brown.
- Change in coordination number - e.g., [Co(H2O)6]2+ is pink whereas [CoCl4]2- is blue.
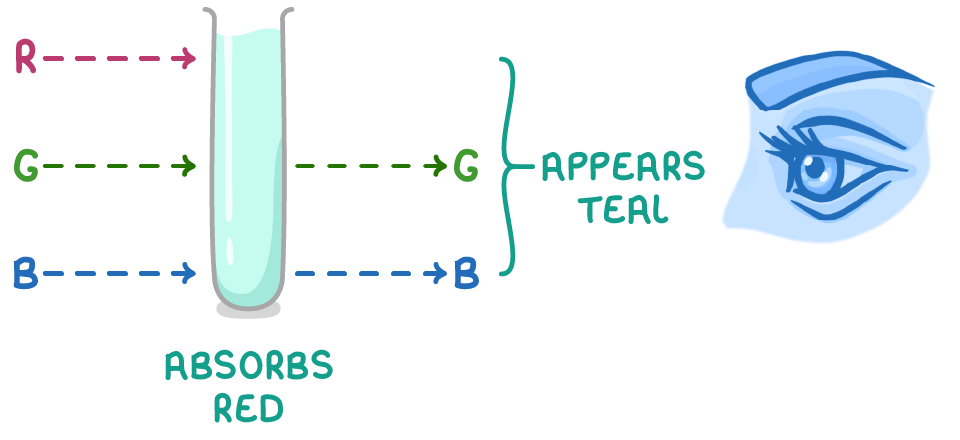
Reflected and transmitted light combine to give the colour
- White light consists of a spectrum of wavelengths from short (violet light) to long (red light).
- When this light hits the complex ion, electrons can jump from ground to excited states by absorbing specific wavelengths of light with energy equal to ΔE.
- The remaining wavelengths of light pass are transmitted or reflected by the complex.
- The transmitted or reflected wavelengths combine to form the colour we actually see for the complex ion.
- This colour is the complementary colour to the wavelengths absorbed.
In the case of [Cu(H2O)6]2+, the complex absorbs red light, and the transmitted frequencies combine to give cyan, the complementary colour to red. As a result, the solution appears teal, a blue-green shade of cyan.
The equation that links the energy gap (ΔE) to the wavelength of light absorbed (λ) is:
ΔE=λhc
Where:
- ΔE = energy gap between ground and excited states (J)
- h = Planck’s constant (6.63 × 10-34 J s)
- c = speed of light in a vacuum (3.00 × 108 m s-1)
- λ = wavelength (m)
Alternatively, the energy gap can be related to the frequency (ν) of the absorbed light using the equation:
ΔE=hν
Where ν is the frequency of the absorbed light (s-1 or Hz).
According to this equation, smaller energy gaps correspond to longer wavelengths of light being absorbed.
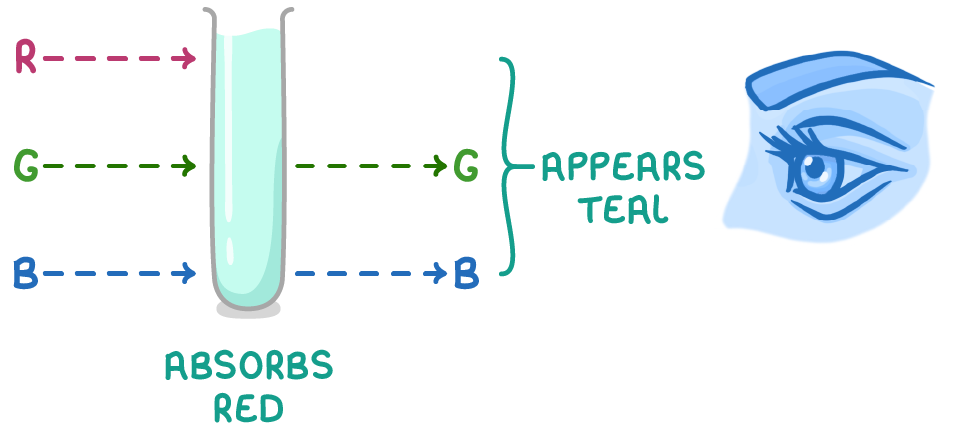
Reflected and transmitted light combine to give the colour
- White light consists of a spectrum of wavelengths from short (violet light) to long (red light).
- When this light hits the complex ion, electrons can jump from ground to excited states by absorbing specific wavelengths of light with energy equal to ΔE.
- The remaining wavelengths of light pass are transmitted or reflected by the complex.
- The transmitted or reflected wavelengths combine to form the colour we actually see for the complex ion.
- This colour is the complementary colour to the wavelengths absorbed.
In the case of [Cu(H2O)6]2+, the complex absorbs red light, and the transmitted frequencies combine to give cyan, the complementary colour to red. As a result, the solution appears teal, a blue-green shade of cyan.