Isomerism in Transition Metal Complexes
This lesson covers:
- What stereoisomerism is
- Optical isomerism in complex ions
- Cis-trans isomerism in complex ions
Stereoisomers and complex ions
Stereoisomers are compounds with the same structural formula but different spatial arrangements of their atoms.
Complex ions can exhibit stereoisomerism because the ligands arranged around the central metal ion can be positioned in different ways.
Optical isomers in complex ions
Optical isomerism is a type of stereoisomerism where molecules exist as non-superimposable mirror images, known as enantiomers.
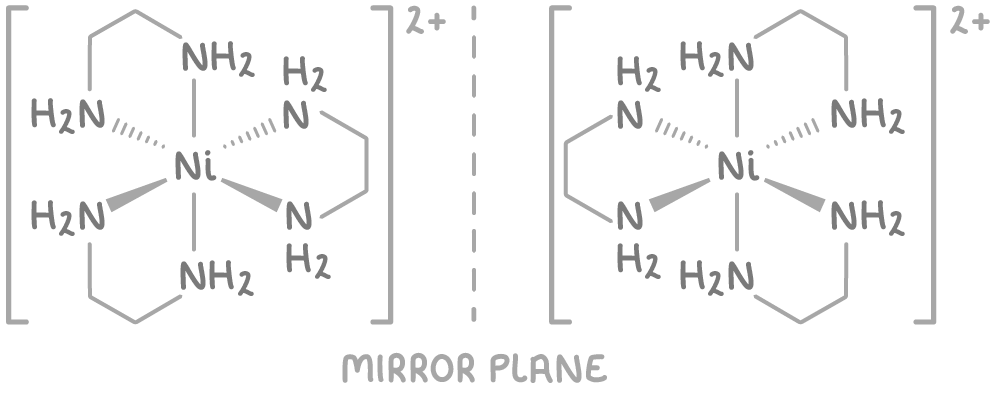
Optical isomerism occurs in octahedral complexes with 3 bidentate ligands.
For example, the complex ion [Ni(NH2CH2CH2NH2)3]2+ can exist as two mirror images that cannot be superimposed:
Cis-trans isomers in complex ions
Cis-trans isomers are types of stereoisomers where identical groups can be positioned either adjacent (cis) or opposite (trans) to each other.
Cis-trans isomerism occurs in:
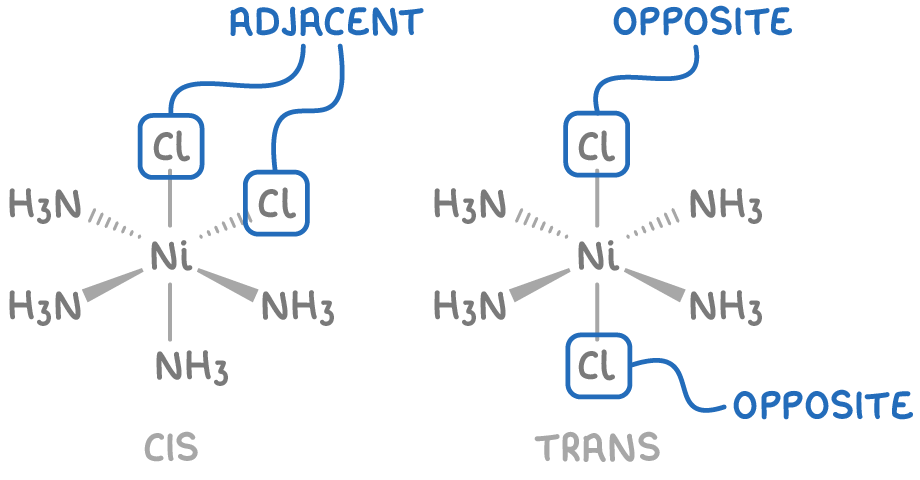
- Octahedral complexes with 4 monodentate ligands of one type and 2 monodentate ligands of another.
For example, in the complex ion Ni(NH3)4Cl2, the Cl- ligands are either adjacent to each other (cis-isomer) or on opposite corners of the octahedron (trans-isomer).
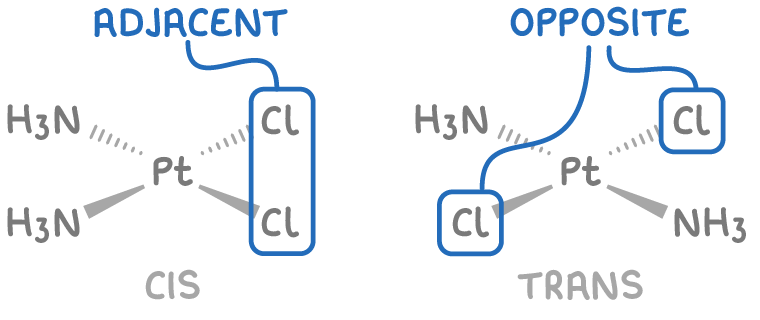
2. Square planar complexes with 2 pairs of monodentate ligands, for example, Pt(NH3)2Cl2.
For example, in the complex ion Pt(NH3)2Cl2, the Cl- ligands are either adjacent to each other (cis-isomer) or diagonally opposite to each other (trans-isomer).