Reactions of Arenes
This lesson covers:
- The mechanism of electrophilic substitution in arenes
- How halogen carriers produce reactive electrophiles
- Nitration of benzene
- Friedel-Crafts acylation reactions to form C-C bonds
Benzene undergoes electrophilic substitution
Electrophilic substitution reactions of benzene involve a hydrogen atom being replaced by an electrophile.
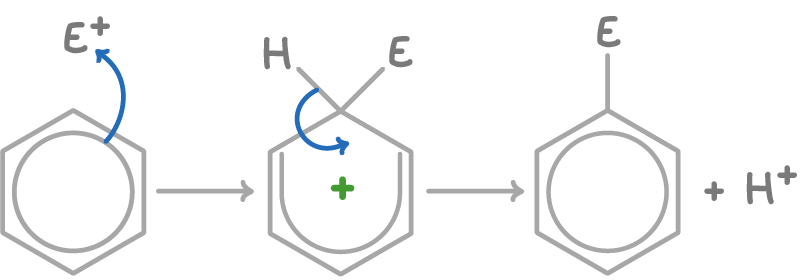
The mechanism unfolds in two stages:
- Addition of the electrophile (E+) to the benzene ring - This breaks the aromaticity and forms a positively charged intermediate.
- Loss of H+ - This step reforms the aromatic benzene ring with the electrophile substituted in place of hydrogen.
Halogen carriers help make good electrophiles
An electrophile has to have a pretty strong positive charge to be able to attack the stable benzene ring. Most compounds just aren't polarised enough — but some can be made into stronger electrophiles using a catalyst called a halogen carrier.
A halogen carrier accepts a lone pair of electrons from a halogen atom on an electrophile. As the lone pair of electrons is pulled away, the polarisation in the molecule increases and sometimes a carbocation forms. This makes the electrophile stronger.
Halogen carriers include aluminium halides, iron halides and iron.
For example, AlCl3 can accept a lone pair from chlorine in CH3COCl, generating the more electrophilic CH3CO+ species:
AlCl3 + CH3COCl ➔ CH3CO+ + AlCl4-
Nitration of benzene
Nitration refers to the electrophilic substitution of a nitro group (-NO2) onto a benzene ring in place of hydrogen. It can be carried out by reacting benzene with concentrated nitric acid in the presence of concentrated sulfuric acid catalyst a temperature of 60°C or less.
The nitration of benzene using HNO3 and H2SO4 occurs as:
- Nitric acid reacts with the sulfuric acid catalyst to generate the electrophilic nitronium (NO2+) ion:
HNO3 + H2SO4 ➔ NO2+ + HSO4- + H2O
- Benzene undergoes electrophilic substitution with the electrophile NO2+, displacing a proton and forming nitrobenzene:
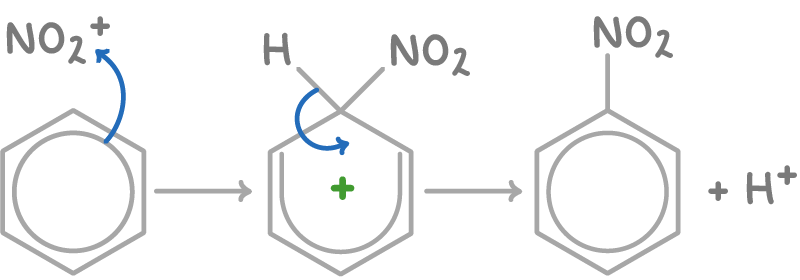
3. The displaced proton reacts with HSO4- to regenerate the H2SO4 catalyst:
H+ + HSO4- ➔ H2SO4
Controlling nitration
Cooling the nitration reaction to less than 60°C ensures that only mononitration occurs, producing nitrobenzene as the major product.
Nitration reactions are useful
- Nitro compounds like nitrobenzene can be catalytically reduced to form aromatic amines. These are key intermediates used to manufacture dyes, drugs and polymers.
- Some nitrated compounds are useful explosives, such as 2,4,6-trinitrotoluene (TNT). The three nitro groups make TNT very unstable and easily detonated.
Friedel-Crafts acylation forms C-C bonds
Friedel-Crafts acylation is a useful reaction for forming C-C bonds in organic synthesis. It is carried out by refluxing benzene with an acyl chloride and a halogen carrier, such as anhydrous aluminium chloride (AlCl3).
In this reaction, an acyl group (-COR) is substituted for a hydrogen atom on the benzene ring. The products of Friedel-Crafts acylation are phenylketones (if the acyl chloride contains an alkyl group) or benzaldehyde (if the acyl chloride is formyl chloride, HCOCl).
Friedel-Crafts acylation mechanism
The acylation of benzene using ethanoyl chloride and AlCl3 proceeds via:
- Formation of the electrophilic acetyl cation (CH3CO+) intermediate:
CH3COCl + AlCl3 ➔ CH3CO+ + AlCl4-
- Electrophilic substitution occurs on benzene with CH3CO+, displacing H+ and forming phenylethanone:
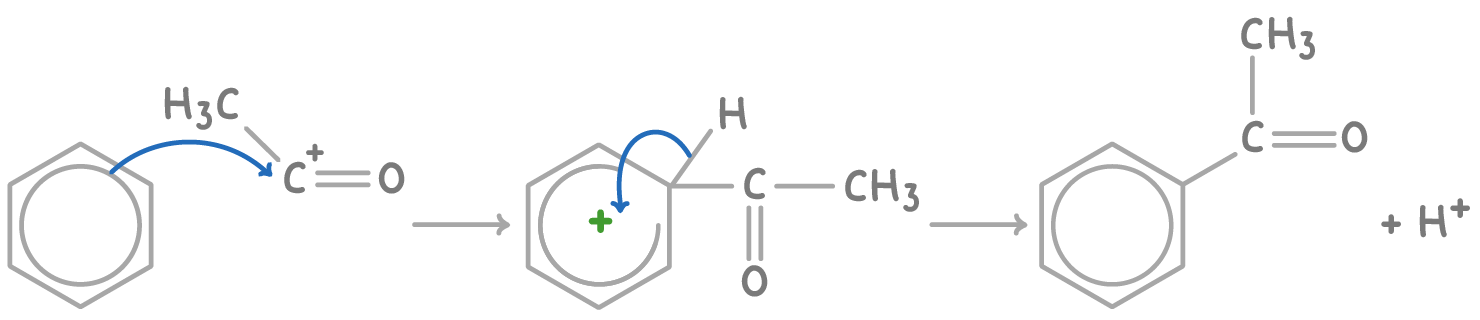
3. The proton reacts with AlCl4-, regenerating the AlCl3 catalyst:
H+ + AlCl4- ➔ AlCl3 + HCl