Redox Equations
This lesson covers:
- Half-equations show oxidation or reduction
- Balancing half-equations
- Combining half-equations into full redox equations
- Disproportionation reactions
Ionic half-equations show electron transfer
Half-equations illustrate the electrons lost or gained during redox reactions.
For example, the half-equation for the oxidation of sodium is:
Na ➔ Na+ + e-.
By combining oxidation and reduction half-equations, you can form the complete redox equation.
Worked example 1 - Writing a redox equation
Write the redox equation for the reaction between magnesium and oxygen to produce magnesium oxide.
Step 1: Write oxygen reduction half-equation
O2 + 4e- ➔ 2O2-
Step 2: Write magnesium oxidation half-equation
Mg ➔ Mg2+ + 2e-
Step 3: Double magnesium half-equation to make the number of electrons the same in both half-equations
2Mg ➔ 2Mg2+ + 4e-
Step 4: Combine half-equations into full redox equation
O2 + 4e- + 2Mg ➔ 2MgO + 4e-
Step 5: Cancel equal electrons on both sides
O2 + 2Mg ➔ 2MgO
Worked example 2 - Writing a redox equation
Write the redox equation for the reaction between aluminium and chlorine to produce aluminium chloride.
Step 1: Write aluminium oxidation half-equation
Al ➔ Al3+ + 3e-
Step 2: Write chlorine reduction half-equation
Cl2 + 2e- ➔ 2Cl-
Step 3: Double aluminium half-equation and triple chlorine half-equation to make the number of electrons the same in both half equations
2Al ➔ 2Al3+ + 6e-
3Cl2 + 6e- ➔ 6Cl-
Step 4: Combine half-equations into full redox equation
2Al + 3Cl2 + 6e- ➔ 2AlCl3 + 6e-
Step 5: Cancel equal electrons on both sides
2Al + 3Cl2 ➔ 2AlCl3
Worked example 3 - Writing a redox equation
Acidified manganate(VII) ions (MnO4-) can be reduced to Mn2+ by Fe2+ ions. Write the full redox equation for this reaction.
Step 1: Write iron oxidation half-equation
Fe2+(aq) ➔ Fe3+(aq) + e-
Step 2: Write manganate reduction half-equation
- Manganate is being reduced. Start by writing this down: MnO4-(aq) ➔ Mn2+(aq)
- To balance the oxygens, add water to the side with fewer oxygen atoms. In this case, add 4H2O to the right-hand side of the equation: MnO4-(aq) ➔ Mn2+(aq) + 4H2O(l)
- To balance the hydrogens, add H+ to the side with fewer hydrogen atoms. In this case, add 8H+ to the left-hand side of the equation: MnO4-(aq) + 8H+(aq) ➔ Mn2+(aq) + 4H2O(l)
- Finally, to balance the charges, add e- to the side with the higher positive charge. In this case, add 5e- to the left-hand side of the equation: MnO4-(aq) + 8H+(aq) + 5e- ➔ Mn2+(aq) + 4H2O(l)
Step 3: Multiply iron half-equation by 5 to make the number of electrons the same in both half-equations
5Fe2+(aq) ➔ 5Fe3+(aq) + 5e-
Step 4: Combine half-equations into full redox equation
MnO4-(aq) + 8H+(aq) + 5e- + 5Fe2+(aq) ➔ Mn2+(aq) + 4H2O(l) + 5Fe3+(aq) + 5e-
Step 5: Cancel equal electrons on both sides
MnO4-(aq) + 8H+(aq) + 5Fe2+(aq) ➔ Mn2+(aq) + 4H2O(l) + 5Fe3+(aq)
To check if the numbers of electrons involved in the equation are correct, we can look at the changes in oxidation numbers of the elements being oxidised and reduced:
- Mn is being reduced from +7 in MnO4- to +2 in Mn2+, a change of -5.
- Fe is being oxidised from +2 in Fe2+ to +3 in Fe3+, a change of +1, but there are 5 Fe2+ ions, so the total change is +5.
Since these oxidation number changes are equal in magnitude but opposite in sign, and they balance the 5 electrons being transferred, we can confirm that the numbers of electrons involved agree with the balanced equation.
Disproportionation is simultaneous oxidation and reduction
In disproportionation reactions, a single element is both oxidised and reduced.
Example:
Cl2 + 2OH− ➔ ClO− + Cl− + H2O
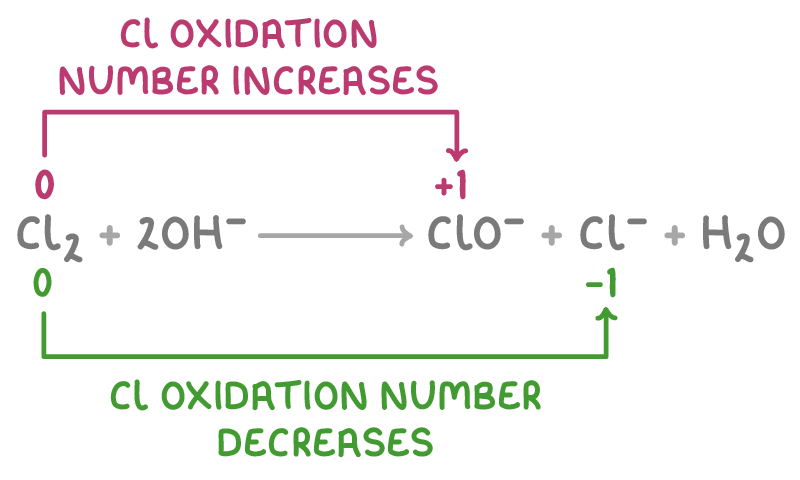
Oxidation number of Cl:
- Increases from 0 (in Cl2) to +1 (in ClO-) so Cl is oxidised.
- Decreases from 0 (in Cl2) to -1 (in Cl-) so Cl is reduced.