Halogenoalkanes - Introduction
This lesson covers:
- How to name halogenoalkanes
- Polarity of the carbon-halogen bond
- Trend in reactivity of halogenoalkanes
Naming halogenoalkanes
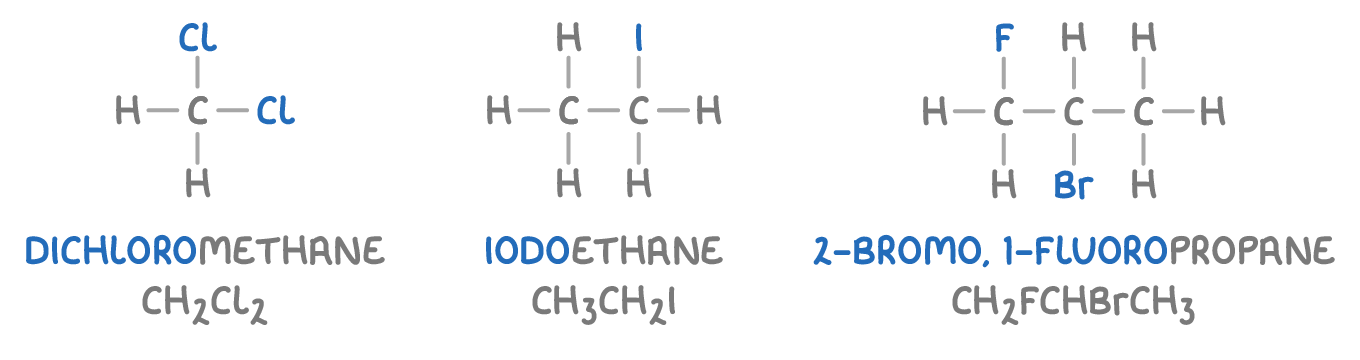
A halogenoalkane is a type of chemical compound where one or more hydrogen atoms in an alkane have been replaced by halogen atoms (like fluorine, chlorine, bromine, or iodine).
To name a halogenoalkane, we use prefixes (like fluoro-, chloro-, bromo-, iodo-) to indicate the type and number of halogen atoms.
Here are some examples of halogenoalkanes:
Polarity of the carbon-halogen bond
In halogenoalkanes, the carbon-halogen bond is polar because halogen atoms have a higher electronegativity than carbon. This causes an uneven distribution of electrons, making the carbon atom partially positively charged (δ+) and the halogen atom partially negatively charged (δ-).
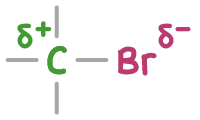
This polarity in the bond makes the carbon atom a target for nucleophiles (electron pair donors). Common nucleophiles include OH-, CN-, NH3, and H2O.
Influence of carbon-halogen bond enthalpy on reaction rates
Bond enthalpy is a measure of bond strength, defined as the energy required to break one mole of bonds between two atoms in the gaseous state. The bond enthalpy of the carbon-halogen bond determines the reactivity of halogenoalkanes.
Halogenoalkanes with lower carbon-halogen bond enthalpies have weaker bonds that require less energy to break. As a result, these compounds react more quickly than those with higher bond enthalpies.
The carbon-halogen bond enthalpy decreases down group 7 due to several factors:
- Increasing atomic radius of the halogens
- Increasing carbon-halogen bond length
- Decreasing electrostatic attraction between bonding electrons and nuclei
As a consequence, the energy needed to break these longer, weaker bonds decreases, leading to faster reaction rates.
Therefore, iodoalkanes (with the weakest carbon-halogen bonds) react the fastest, while fluoroalkanes (with the strongest bonds) react the slowest.
| Bond | Bond enthalpy (kJ mol-1) |
|---|---|
| C–F | 467 |
| C–Cl | 346 |
| C–Br | 290 |
| C–I | 228 |