Determining the Rate Equation
This lesson covers:
- Designing experiments to determine the rate equation
- The initial rates method
- Continuous monitoring methods
- Interpreting rate-concentration graphs
Performing rate experiments to determine reaction orders
To determine the reaction order with respect to the concentration of a specific reactant, you can use two main methods:
- Initial rates method - This involves measuring how the initial rate changes when you alter the initial concentration of the reactant in question.
- Continuous monitoring - This involves tracking the change in concentration of the reactant over time and plotting a rate-concentration graph.
In both strategies, ensure that the concentrations of any other reactants remain substantially constant (in large excess) throughout the experiment. This approach helps ensure that any observed changes in rate are solely due to the reactant being studied.
Using the initial rates method to work out rate equations
The initial rate of a reaction refers to the rate at the very beginning of the reaction (time = 0) and is found by calculating the gradient of the tangent to the concentration-time graph at t = 0.

The steps involved in the initial rates method are:
- Perform the reaction several times, each time changing the initial concentration of one reactant while keeping the others constant.
- Calculate the initial rate from the concentration-time data for each experiment.
- Analyse how the initial rates change with varying initial concentrations to deduce the order of the reaction with respect to each reactant.
- Use these findings to assemble the overall rate equation.
Worked example 1 - Determining the rate equation using initial rates
Consider the reaction below, carried out at a constant temperature.
2NO(g) + O2(g) ➔ 2NO2(g)
| Experiment | [NO] (mol dm-3) | [O2] (mol dm-3) | Initial rate (mol dm-3 s-1) |
|---|---|---|---|
| 1 | 0.05 | 0.05 | 0.020 |
| 2 | 0.10 | 0.05 | 0.080 |
| 3 | 0.05 | 0.10 | 0.040 |
Determine the rate equation for this reaction.
Step 1: Analyse NO concentration change
Between experiments 1 and 2, the concentration of [NO] doubles while [O2] remains constant, and the rate quadruples. This suggests the reaction is second order with respect to [NO].
Step 2: Analyse O2 concentration change
Between experiments 1 and 3, doubling the concentration of [O2] while keeping [NO] constant doubles the rate, indicating first-order dependence on [O2].
Step 3: Write the rate equation
rate = k[NO]2[O2]
Worked example 2 - Calculating the rate constant using initial rates
Using the same reaction and data from the previous worked example 1, calculate the rate constant (k), including its units.
Step 1: Rearrange rate equation
First, rearrange the rate equation to solve for k.
k =[NO]2[O2]rate
Step 2: Substitution and correct evaluation
Substitute values from one of the experiments into the equation to find k. For example, substituting values from experiment 1:
k =(0.05)2×0.050.020=160
Step 3: Determine the units
Given that the rate is expressed in mol dm-3 s-1 and the concentration cubed in (mol dm-3)3, solving for k in the equation results in units of mol-2 dm6 s-1.
Therefore the rate constant for the reaction above is 160 mol-2 dm6 s-1.
Using continuous monitoring methods to work out rate equations
Rather than finding initial rates, some reactions can be continuously monitored as they proceed to completion. The amounts of reactant or product are determined at regular intervals to see how the rate varies over the course of the reaction.
Numerous methods can be used depending on what property changes during the reaction:
- Measuring the volume of gas evolved.
- Measuring the mass loss as a gas escapes.
- Measuring pH changes.
- Using colorimetry to quantify colour changes.
Determining reaction order from rate-concentration graphs
The shape of a rate-concentration graph can reveal the reaction order with respect to a particular reactant.
To construct a rate-concentration graph from experimental data, follow these steps:
- Begin with a concentration-time graph for the reactant of interest.
- Calculate the rate (gradient) at various points along the concentration-time curve.
- For linear plots, the gradient is constant and can be easily determined.
- For curved plots, draw tangent lines at selected points to determine the gradient at each point.
- Plot the rate (y-axis) against the corresponding concentration (x-axis) for each point.
- Fit a smooth curve or line through the resulting data points.
The shape of the resulting rate-concentration graph reveals the reaction order with respect to the reactant:
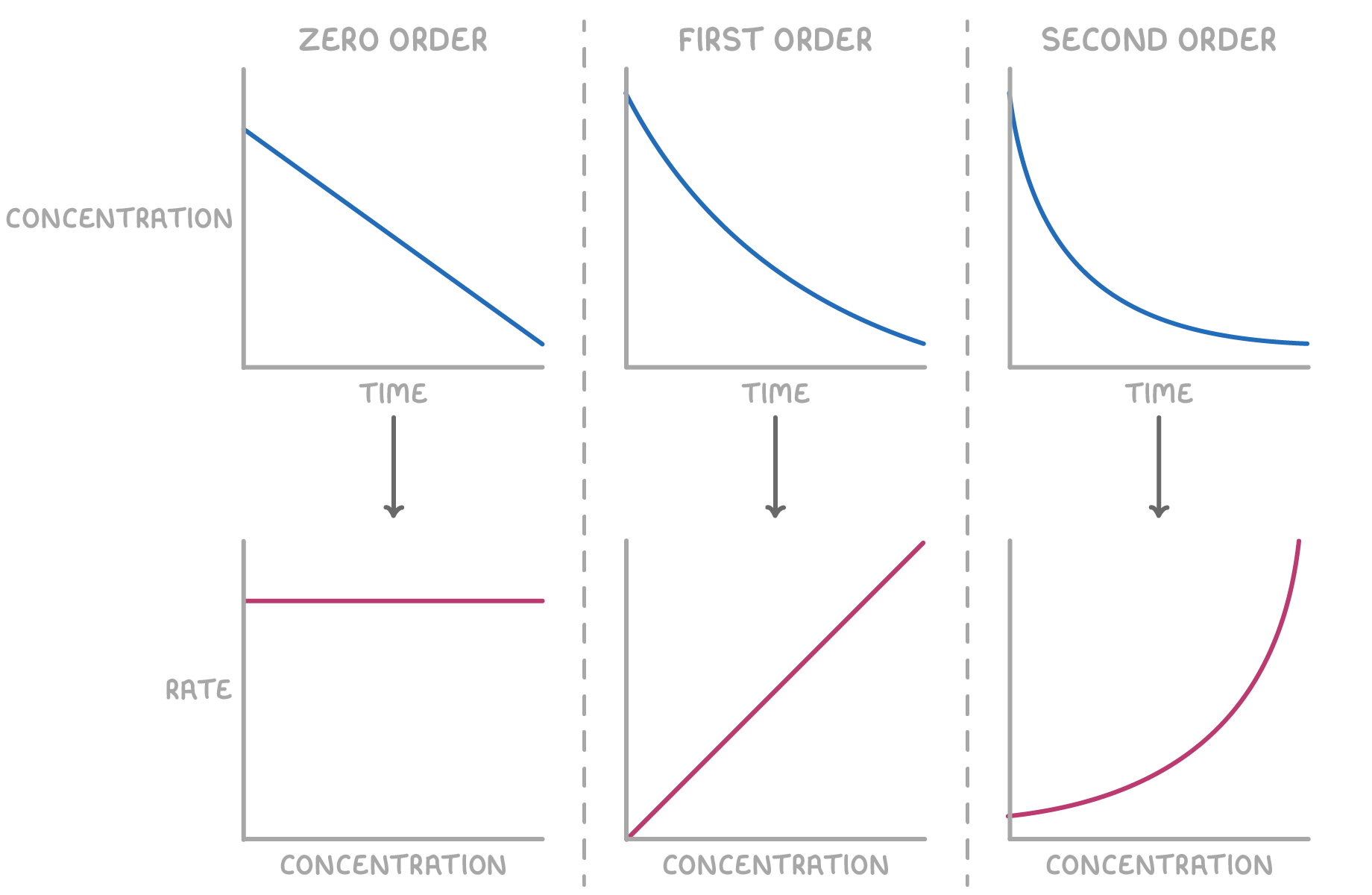
- Zero order - A horizontal line indicates that the reaction rate is independent of the reactant concentration.
The rate equation is: rate = k
- First order - A straight line passing through the origin signifies that the reaction rate is directly proportional to the reactant concentration.
The rate equation is: rate = k[X]
- Second order - A curved plot indicates that the reaction rate is proportional to the square of the reactant concentration.
The rate equation is: rate = k[X]2
In these equations, k represents the rate constant, and [X] denotes the concentration of reactant X.