Condensation Polymers
This lesson covers:
- How condensation polymers form through elimination reactions
- Polyesters, polyamides, and polypeptides
- Working out monomer and repeat unit structures
- Intermolecular forces in condensation polymers
- Comparing addition and condensation polymerisation
Condensation polymerisation involves elimination reactions
Condensation polymerisation reactions lead to the formation of long polymer chains through the joining of monomers, accompanied by the elimination of small molecules like water (H2O) or hydrogen chloride (HCl).
Key features of condensation polymers:
- They are formed from reactions between monomers that contain at least two functional groups.
- The functional groups of the monomers join up, creating links between the repeat units.
- A small molecule, such as water or hydrogen chloride, is eliminated each time a link forms.
Common types of condensation polymers are:
- Polyesters
- Polyamides
- Polypeptides
Polyesters
Polyesters are a type of condensation polymer formed from the reaction between dicarboxylic acids (or derivatives such as acyl chlorides) and diols. The carboxyl (–COOH) and hydroxyl (–OH) groups react, forming ester links in the polymer chain.
An example of a polyester is Terylene, which is synthesised from benzene-1,4-dicarboxylic acid and ethane-1,2-diol:
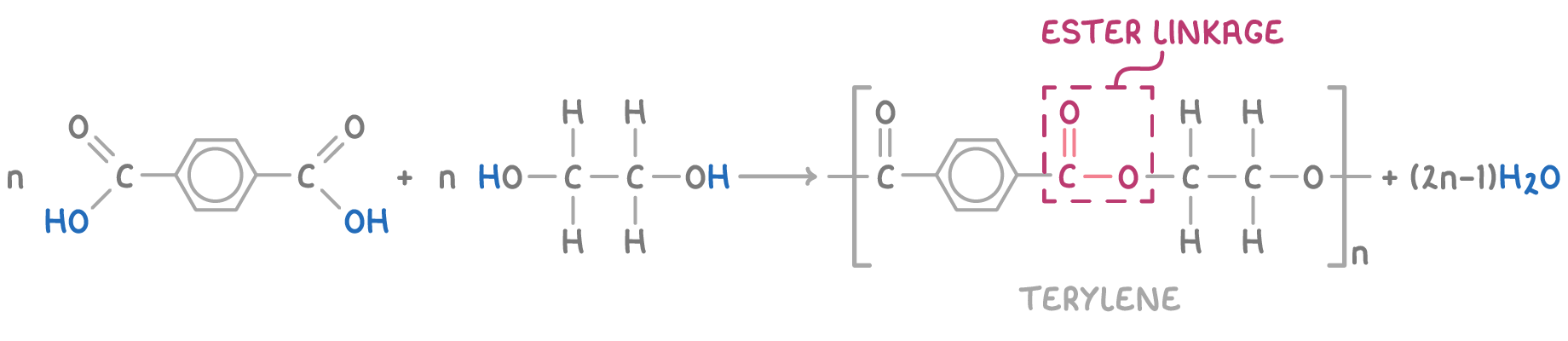
Terylene is commonly used for plastic bottles and containers, as well as fibres for clothing and fabrics.
Polyamides
Polyamides are another type of condensation polymer, formed from dicarboxylic acids (or derivatives such as acyl chlorides) and diamines. The carboxyl and amine groups react, forming amide links within the polymer chain.
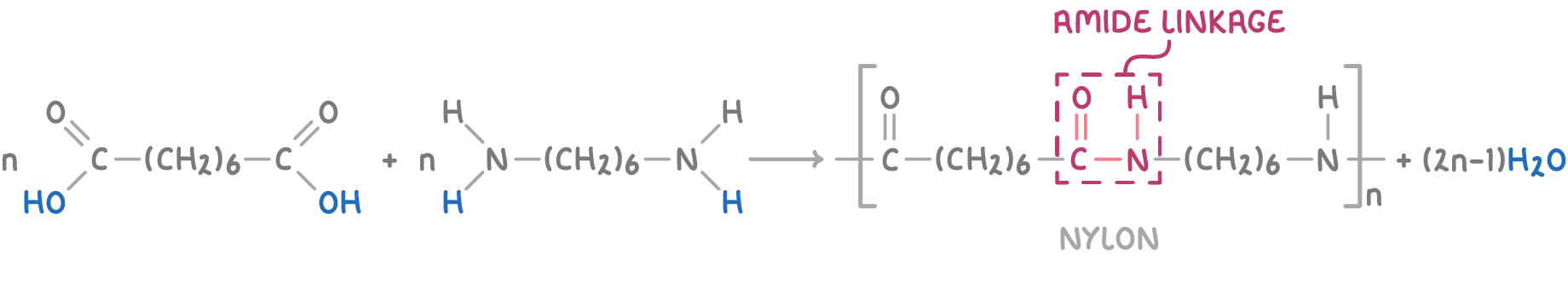
Examples of polyamides include:
- Nylon - Formed from hexanedioic acid and 1,6-diaminohexane. Nylon is commonly used for fibres in clothing, ropes and parachutes.
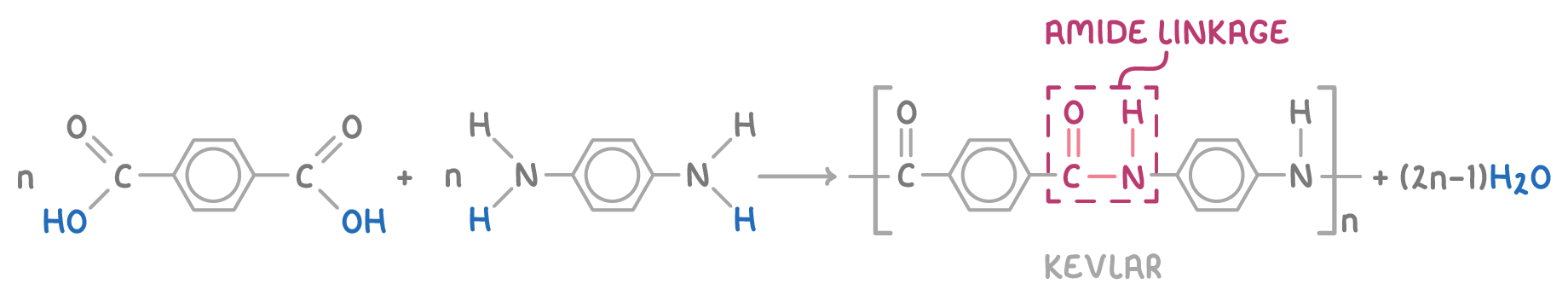
- Kevlar - Formed from benzene-1,4-dicarboxylic acid and 1,4-diaminobenzene. Kevlar is used for bulletproof vests and sports equipment.
Polypeptides
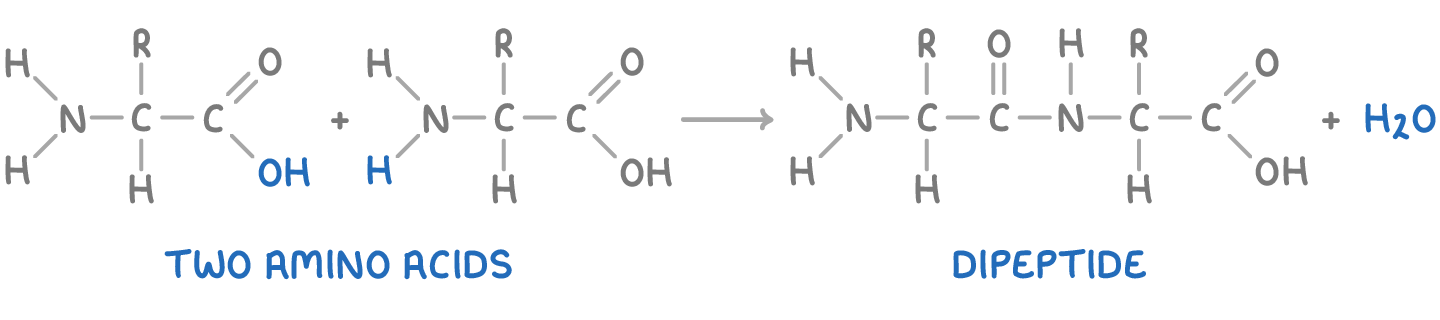
Polypeptides are condensation polymers formed from amino acids. The carboxyl group of one amino acid reacts with the amine group of another, forming a peptide (amide) link.
Long chains of polypeptides, with more than 50 amino acid units, are called proteins. The sequence and shape of proteins are determined by the order of amino acids in their polypeptide chain.
For example, the formation of a dipeptide, where R represents the amino acid side group, is illustrated below:
Working out monomer and repeat unit structures
Drawing monomers from a polymer
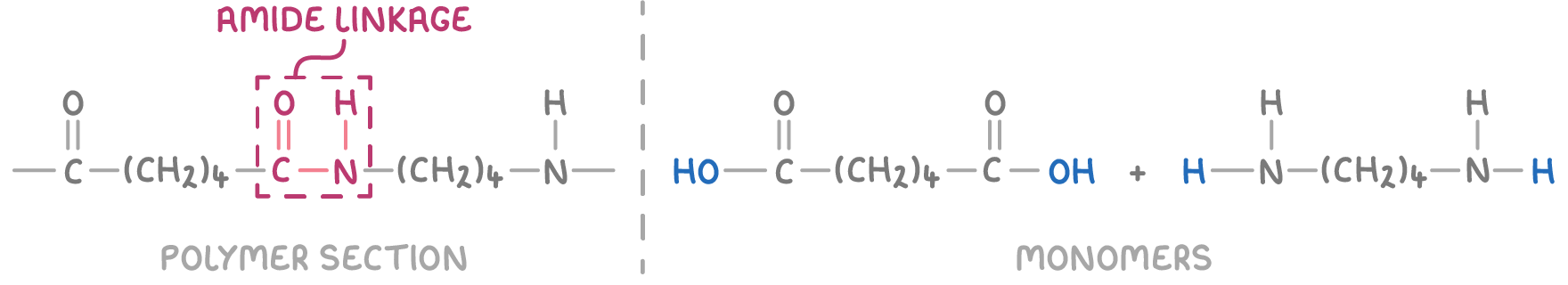
To deduce the monomers that form a condensation polymer, follow these steps:
- Identify the amide (HN-CO) or ester (CO-O) linkage between repeat units.
- Cut the link in the middle to separate the polymer chain.
- Add an H atom or -OH group to both ends of the molecules. H atoms are added to N or O atoms, and -OH groups to C atoms.
For example, the monomers that form a particular polyamide can be identified from its polymer structure:
Drawing repeat units from monomers
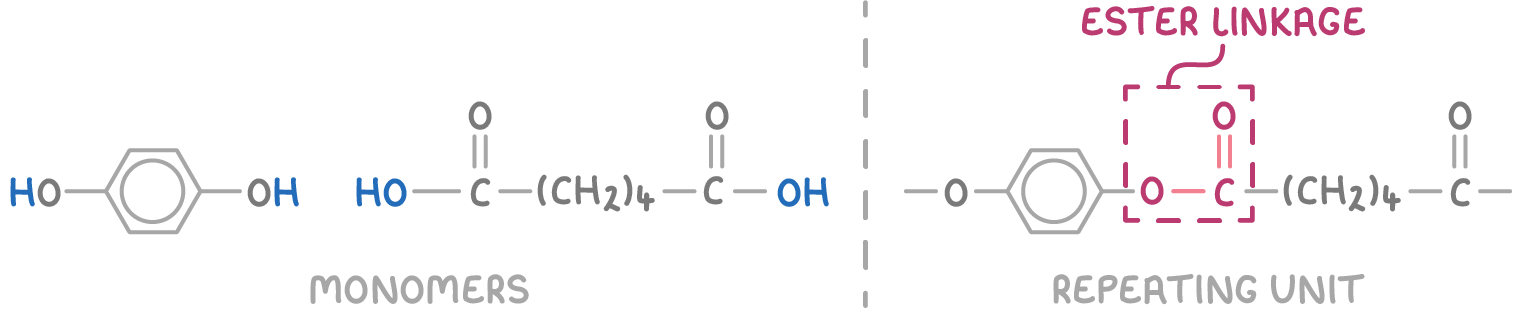
To draw the repeat unit from condensation monomers, follow these steps:
- Place the structures of the two monomers side by side.
- Remove an H atom from one monomer (e.g., diol or diamine) and an OH group from the other (e.g., dicarboxylic acid), forming a H2O molecule as a by-product. For diacyl chlorides, remove Cl instead of OH, forming HCl.
- Connect the monomers through either an amide or ester linkage, depending on their functional groups.
- To indicate continuity, remove another H atom and OH/Cl group from the opposite ends.
The repeating unit of a polyester, for instance, can be deduced from its monomers' structures:
Polar groups determine polymer properties
Condensation polymers feature polar covalent bonding, such as amide or ester groups, along their chain backbone.
These include:
- Amide (peptide) links (-NHCO-) in polyamides and polypeptides.
- Ester links (-COO-) in polyesters.
These polar links lead to strong intermolecular forces.
Intermolecular forces:
- Permanent dipole-dipole forces between polarised groups.
- Hydrogen bonding between C=O, N-H, and O-H groups.
Additionally, induced dipole-dipole forces arise between temporary dipoles on the polymer chains.
By contrast, addition polymers, which consist only of nonpolar C-C and C-H bonds, rely on weaker induced dipole-dipole forces between chains.
Effects on properties
The presence of stronger intermolecular forces makes condensation polymers:
- More rigid and less flexible.
- Stronger, with higher melting and boiling points compared to addition polymers.
Comparing addition and condensation polymerisation
The mechanisms of addition and condensation polymerisation differ significantly:
| Polymerisation type | Addition | Condensation |
|---|---|---|
| Monomers used | Alkenes | Carboxylic acids, acyl chlorides, amines, alcohols, amino acids |
| Polymer chain | Continuous chain of carbon atoms | Contains ester or amide bonds |
| Products formed | Polymer only | Polymer and small molecule (e.g. H2O, HCl) |
Addition polymers are synthesised from alkene monomers, which contain C=C double bonds, forming chains composed solely of carbon atoms.
Condensation polymers, on the other hand, are synthesised from monomers containing functional groups such as -COOH, -NH2, and -OH, leading to chains that incorporate ester and/or amide bonds.