Catalysis
This lesson covers:
- How transition metal catalysts work by changing oxidation states
- The difference between heterogeneous and homogeneous catalysts
- Vanadium(V) oxide as a heterogeneous catalyst
- How impurities can poison heterogeneous catalysts
- Fe2+ and Mn2+ ions as homogeneous catalysts
Transition metals as catalysts
Many transition metals and their compounds act as good catalysts because:
- They have multiple stable oxidation states - They have multiple vacant d orbitals with similar energies, allowing them to transition between different charges.
- They readily forming dative bonds with ligands - Their vacant d orbitals can form coordinate bonds with ligands, facilitating electron transfer.
This allows them to accept or donate electrons during a reaction, significantly lowering activation energies and increasing reaction rates.
For example, vanadium(V) oxide is used as a catalyst in the Contact process for the manufacture of sulfuric acid:
V2O5 catalyses the oxidation of SO2 to SO3 by being reduced itself from vanadium(V) to vanadium(IV) in the process:
- SO2(g) + V2O5(s) ➔ SO3(g) + V2O4(s)
The V2O4 is then re-oxidised back to V2O5 by oxygen, regenerating the catalyst:
- V2O4(s) + 1⁄2O2(g) ➔ V2O5(s)
Heterogeneous vs. homogeneous catalysts
Catalysts can be classified into two different types depending on their relative phase compared to the reactants.
Heterogeneous catalysts:
- Exist in a different phase from the reactants (typically solid catalysts for gaseous or liquid reactants).
- Catalyse reactions by adsorption of reactants onto active sites located on the surface of the catalyst.
- Support mediums with high surface area are used as a base to disperse the catalyst particles and maximise exposed surface area.
- Increasing the surface area to volume ratio increases the number of exposed active sites which leads to a lower activation energy.
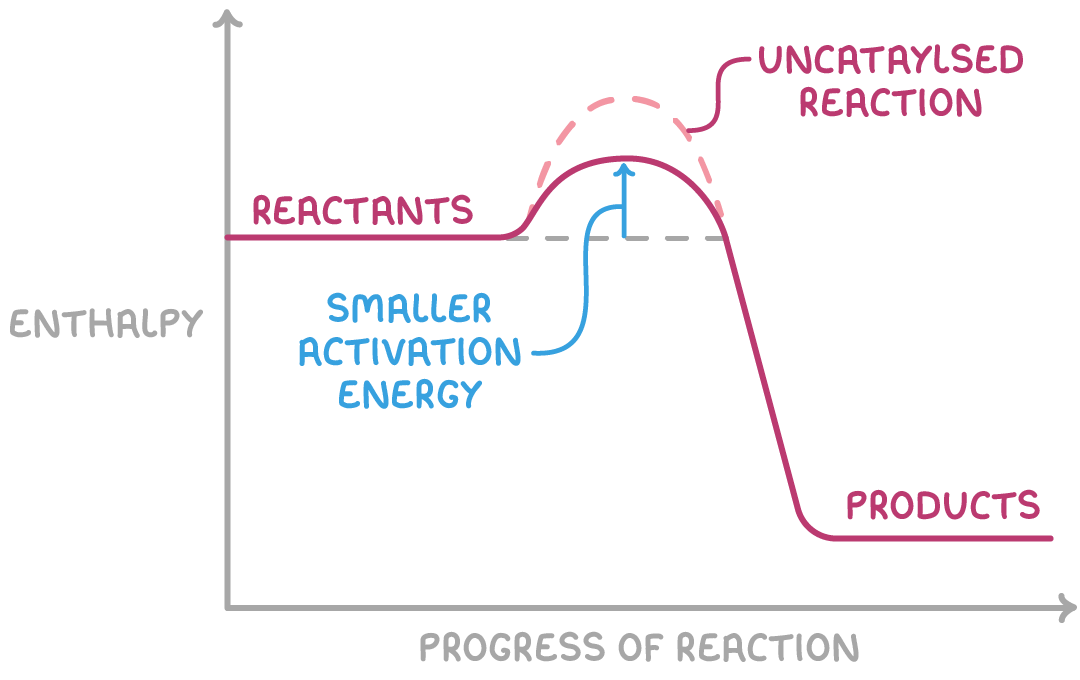
The enthalpy profile diagram demonstrates how the presence of a heterogeneous catalyst provides an alternative reaction pathway with a lower activation energy barrier compared to the uncatalysed reaction.
Examples of heterogeneous catalysts include:
- Iron in the synthesis of ammonia from nitrogen and hydorgen in the Haber process.
- Vanadium(V) oxide (V2O5) in the production of sulfur trioxide from sulfur dioxide and oxygen in the Contact process.
Homogeneous catalysts:
- Exist in the same phase as reactants (typically aqueous ionic catalysts and reactants).
- Catalyse reactions by forming intermediate compounds with reactants, which decompose to products.
- This two-step mechanism lowers activation energies compared to the uncatalysed reaction.
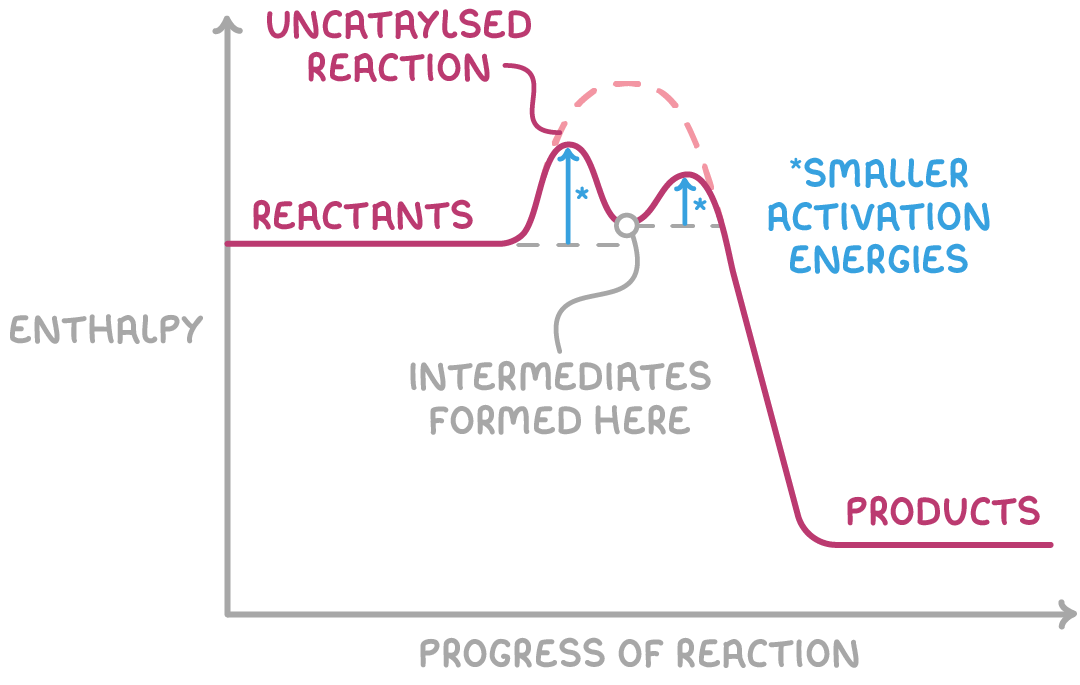
The enthalpy profile diagram illustrates the formation of intermediate compounds from the reactants, which then decompose to yield the products. This two-step mechanism effectively lowers the overall activation energy compared to the uncatalysed reaction pathway.
Examples of homogeneous catalysts include:
- Fe2+ ions catalyse the reaction between S2O82- and I- ions in solution.
- Mn2+ ions catalyse the reaction between MnO4- and C2O42- ions in solution.
V2O5 catalyses SO2 oxidation in the Contact process
In the Contact process for producing sulfur trioxide (SO3), vanadium(V) oxide (V2O5) catalyses the oxidation of sulfur dioxide (SO2) by oxygen gas.
The mechanism involves:
- V2O5 oxidises SO2 to SO3, being reduced from vanadium(V) to vanadium(IV) in the process:
SO2(g) + V2O5(s) ➔ SO3(g) + V2O4(s)
- The V2O4 is then oxidised back to V2O5 by oxygen, regenerating the catalyst:
V2O4(s) + 1⁄2O2(g) ➔ V2O5(s)
Poisoning of heterogeneous catalysts
Catalyst poisoning happens when impurities in a reaction bind to the surface of a heterogeneous catalyst, blocking access to the active sites where reactants would normally react.
The consequences of catalyst poisoning are:
- Reduces available catalyst surface area.
- Lowers reaction rate.
- Increases operating costs due to less product output for a given catalyst mass and energy input.
- Can necessitate costly catalyst replacement.
For example, sulfur poisons the iron catalyst in the Haber process - the hydrogen from natural gas contains sulfur impurities that adsorb onto the iron catalyst surface as iron sulfide.
Fe2+ catalyses the reaction between S2O82- and I-
The redox reaction between iodide ions (I-) and peroxodisulfate ions (S2O82-) proceeds very slowly uncatalysed because both ions are negatively charged. The ions repel each other, so few collisions occur to react.
S2O82-(aq) + 2I-(aq) ➔ I2(aq) + 2SO42-(aq)
But when Fe2+ ions are added as a catalyst, the reaction rate increases dramatically because the Fe2+ and S2O82- ions have opposite charges and are attracted to each other.
The mechanism involves:
- Fe2+ ions are oxidised to Fe3+ by S2O82-:
S2O82-(aq) + 2Fe2+(aq) ➔ 2Fe3+(aq) + 2SO42-(aq)
- The Fe3+ ions then oxidise I- to I2, regenerating Fe2+:
2Fe3+(aq) + 2I-(aq) ➔ I2(aq) + 2Fe2+(aq)
Since each step involves a positive and negative ion, there is no repulsion to slow the reaction.
The iodine product can be detected by adding starch solution, which turns blue-black in the presence of iodine.
Mn2+ autocatalyses the reaction between MnO4- and C2O42-
The reaction between permanganate ions (MnO4-) and oxalate ions (C2O42-) is an autocatalytic reaction. Autocatalysis occurs when a product of the reaction acts as a catalyst, speeding up the reaction as the product accumulates. In this case, Mn2+ is the autocatalytic product that accelerates the reaction.
The overall reaction is:
5C2O42-(aq) + 2MnO4-(aq) + 16H+(aq) ➔ 10CO2(g) + 2Mn2+(aq) + 8H2O(l)
The mechanism showing Mn2+ autocatalysis is:
- Mn2+ catalyses the reaction by first reacting with MnO4- to form Mn3+ ions:
MnO4-(aq) + 4Mn2+(aq) + 8H+(aq) ➔ 5Mn3+(aq) + 4H2O(l)
- The Mn3+ ions then react with C2O42- to regenerate Mn2+:
2Mn3+(aq) + C2O42-(aq) ➔ 2Mn2+(aq) + 2CO2(g)