Fractional Distillation of Crude Oil
This lesson covers:
- The composition of crude oil
- The concept of fractional distillation
- How fractional distillation separates crude oil into useful fractions
- The key properties and uses of the different fractions obtained
Crude oil is a mixture of hydrocarbons
Crude oil, also known as petroleum, is a complex mixture of hydrocarbons extracted from underground oil reservoirs. The main components are alkanes, which are saturated hydrocarbons consisting of carbon and hydrogen atoms in straight chains.
Crude oil contains alkanes ranging from:
- Small molecules like pentane (C5H12)
- To larger alkanes over 50 carbons long
The varying lengths of the alkane chains result in a broad range of boiling points in crude oil, from -162°C to more than 350°C. This range of boiling points is crucial for the separation of crude oil into different components by fractional distillation.
Fractional distillation of crude oil
Fractional distillation is a technique used to separate hydrocarbon components of crude oil based on their boiling points. The process:
- Involves heating crude oil to about 350°C in a furnace, causing it to vaporise.
- The vaporised components are then separated by their ability to condense at different temperatures in a tall column known as a fractionating column.
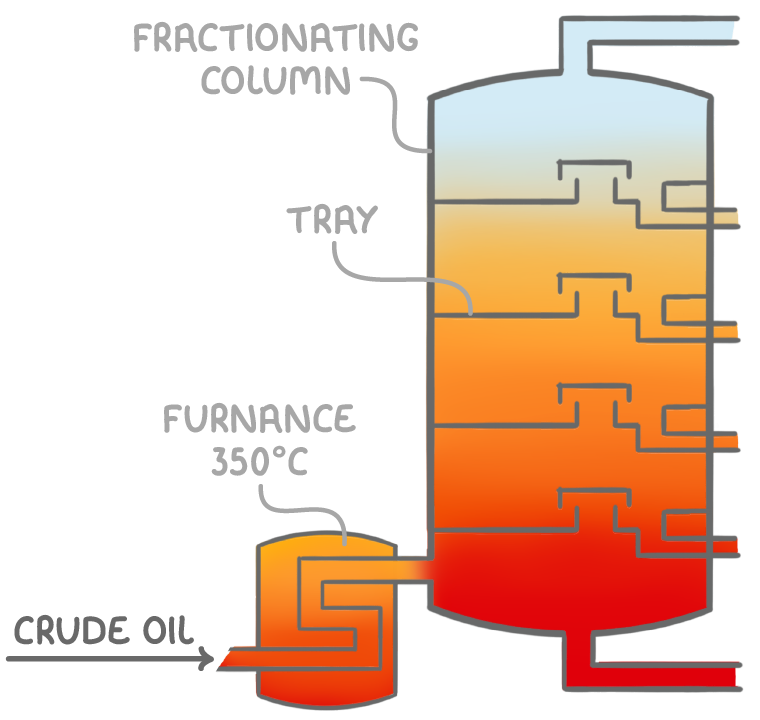
This technique is effective because the various hydrocarbon components have different boiling points related to their chain lengths. As the vapour mixture rises and cools in the column, fractions with higher boiling points condense first, while heavier residues remain at the bottom.
How fractional distillation works
The key steps in fractional distillation are:
- Crude oil is heated to above 350°C in a furnace to vaporise the hydrocarbon mixture.
- These vapours enter a fractionating column with temperatures higher at the bottom (~350°C) and lower near the top (~40°C).
- As the hot vapours rise through the column, they cool down. When the vapour temperature drops below the boiling point of a hydrocarbon in the mixture, it condenses from gas to liquid on the tray surface.
- The condensed hydrocarbon liquids that accumulate on each tray are drawn off at specific intervals as fractions (mixtures of hydrocarbons with similar boiling points).
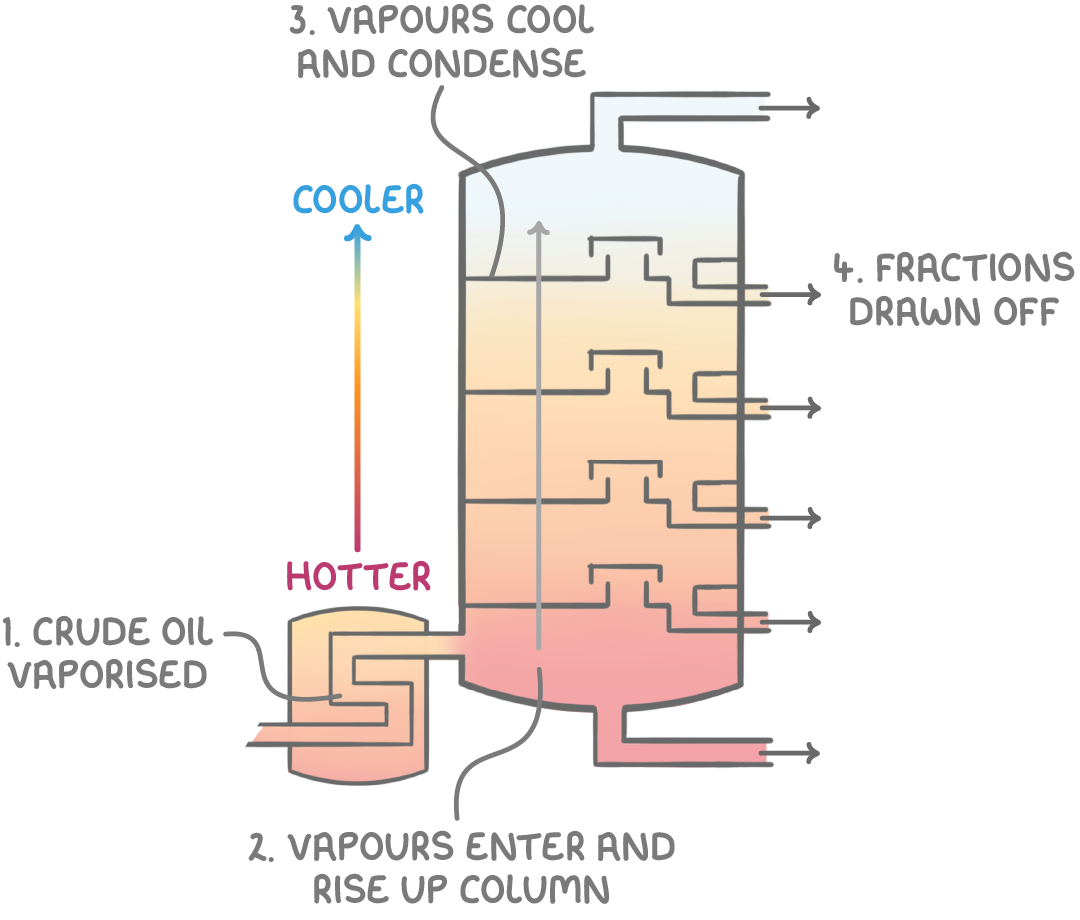
The smallest hydrocarbons, with the lowest boiling points, do not condense at all and remain gas gases at the top. Larger molecules, over 50 carbons, have the highest boiling points and condense near the bottom of the comumn to form a tar-like residue.
Key properties of fractions
The separated fractions exhibit consistent trends in key properties as you move down the fractionating column, which make them suitable for specific uses.
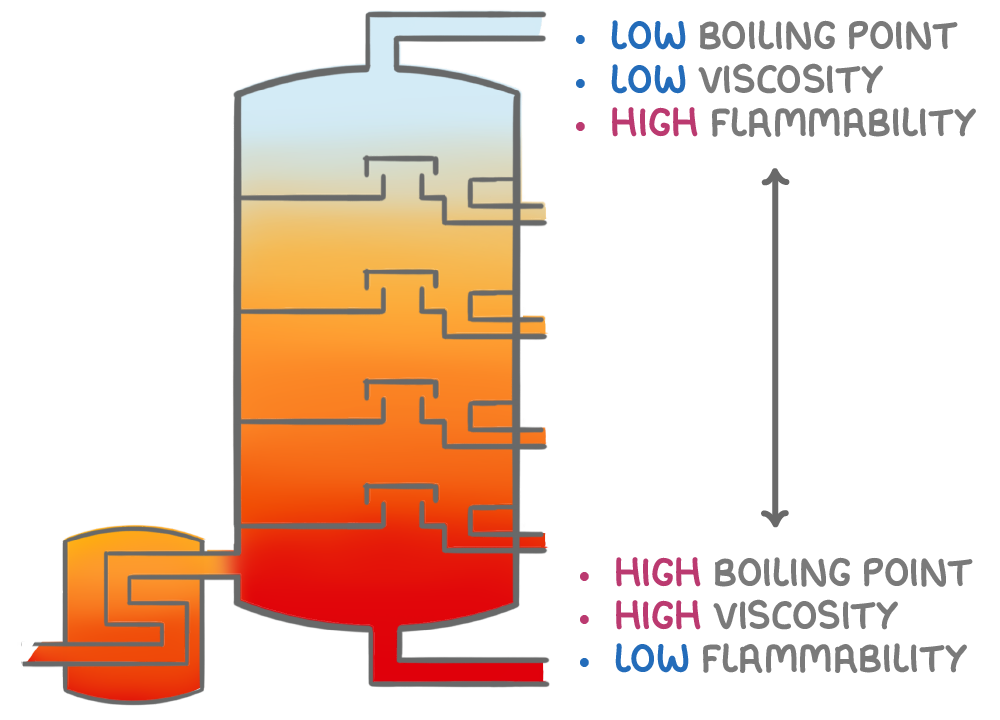
Boiling point - The boiling point increases progressively down the column as the alkane chain length increases in each successive fraction. The lightest gases boil below 0°C, while the undistillable residue requires temperatures over 500°C.
Viscosity - Viscosity steadily increases as you move down the column. Very light distillates, like gasoline or jet fuel, flow freely. Heavier fractions collected towards the bottom demonstrate progressively higher viscosities.
Flammability - Flammability decreases down the fractionating column. Light gases and short-chain hydrocarbons at the top ignite readily, making them excellent fuels due to their high flammability and volatility. In contrast, heavy fractions and long-chain hydrocarbons at the bottom are less flammable and volatile, making them harder to combust and less suitable as fuels.
The table below summarises the main fractions obtained from crude oil and their uses:
| Fraction | Number of carbons | Uses |
|---|---|---|
| Gases | 1 - 4 | Liquefied petroleum gas (LPG), camping gas |
| Petrol (gasoline) | 5 - 12 | Fuel for vehicles |
| Naphtha | 7 - 14 | Petrochemical feedstock |
| Kerosene (paraffin) | 11 - 15 | Jet fuel, heating fuel |
| Gas Oil (diesel) | 15 - 19 | Diesel engines, heating |
| Mineral Oil | 20 - 30 | Lubricants |
| Fuel Oil | 30 - 40 | Ships, power stations |
| Wax, Grease | 40 - 50 | Candles, lubricants |
| Bitumen | 50+ | Roofing, roads |