Reaction Mechanisms
This lesson covers:
- Types of bond fission
- Using reaction mechanisms and curly arrows
- Classifying organic reactions by type
- Differentiating between nucleophilic, electrophilic, and radical reagents
Bond fission
When a covalent bond breaks, it undergoes one of two processes: heterolytic or homolytic fission:
Heterolytic fission
- Involves uneven cleavage of the shared electron pair within the bond.
- One atom retains both bonding electrons, acquiring a negative charge and becoming an anion.
- The other atom loses both electrons, gaining a positive charge and becoming a carbocation.
- This type of fission is common in reactions occurring in solution or involving ions or polar molecules.
For example: X-Y ➔ X+ + Y-
Homolytic fission
- Involves even cleavage of the shared electron pair within the bond.
- Each atom retains one electron from the bonding pair, forming two neutral radicals with unpaired electrons (depicted as a dot in equations and mechanisms).
- Radicals are very reactive due to their unpaired electrons.
- Commonly observed in gas-phase reactions and with nonpolar substances.
For example: X-Y ➔ X• + Y•
Reaction mechanisms
Reaction mechanisms depict the sequential steps through which reactants transform into products. They employ curly arrows to represent the movement of electron pairs during the formation and breaking of bonds.
The steps for drawing curly arrows are:
- Initiate the arrow at the bond or lone electron pair, indicating the origin of the electrons.
- Conclude the arrow pointing towards the new location of the electrons, signifying either the formation of a new bond or the transfer onto an atom.
- Ensure the arrow direction accurately represents the electron movement in the bonding processes.
For example, the mechanism for the reaction CH3Cl + NaOH ➔ CH3OH + NaCl is depicted below:
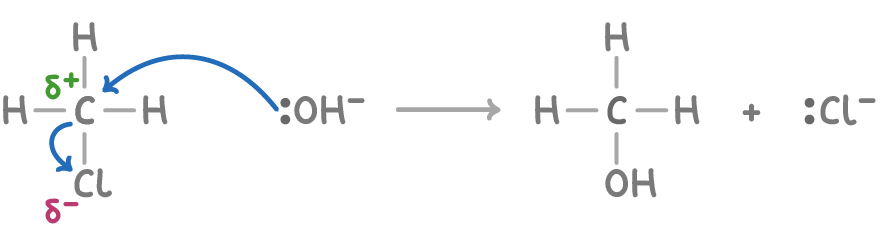
Classifying organic reactions
Organic reactions can be categorised into several key types:
- Addition - The joining of two or more molecules to form a larger molecule.
- Elimination - A small group of atoms detaches from a larger molecule.
- Substitution - One atom or group is replaced by another.
- Hydrolysis - A molecule splits by incorporating H+ and OH- from water.
- Oxidation - A process where a species loses electrons to an oxidising agent, represented by [O] in equations.
- Reduction - A process where a species gains electrons from a reducing agent, represented by [H] in equations.
Classifying reagents
Understanding the type of reagent is crucial for predicting the likely products of a reaction:
- Nucleophiles - Electron pair donors. They contain lone pairs or negative charges and can donate those electrons during reactions. Examples include halide ions, oxygen, and nitrogen atoms.
- Electrophiles - Electron pair acceptors. They are electron deficient and can accept electron pairs during reactions. Examples include H+ ions, alkenes, and benzene rings.
- Radicals - Species with one (or more) unpaired electrons. Their unpaired electron makes them highly reactive. They do not donate/accept electron pairs but can react with most chemical bonds, including non-polarised C-C and C-H bonds. Examples include Cl• and CH2Cl• radicals.