Bonding and Physical Properties
This lesson covers:
- The 4 types of crystal lattice structure
- The physical properties of each crystal type
- Explaining the properties in terms of structure and bonding
- Predicting structure from physical properties
The 4 types of crystal lattice structure
There are 4 main types of crystal lattice structure:
- Ionic - e.g. NaCl
- Metallic - e.g. Mg
- Simple molecular - e.g. I2 and H2O.
- Giant covalent - e.g. diamond and graphite.
The table below illustrates the key structural features of the four main types of crystal lattice:
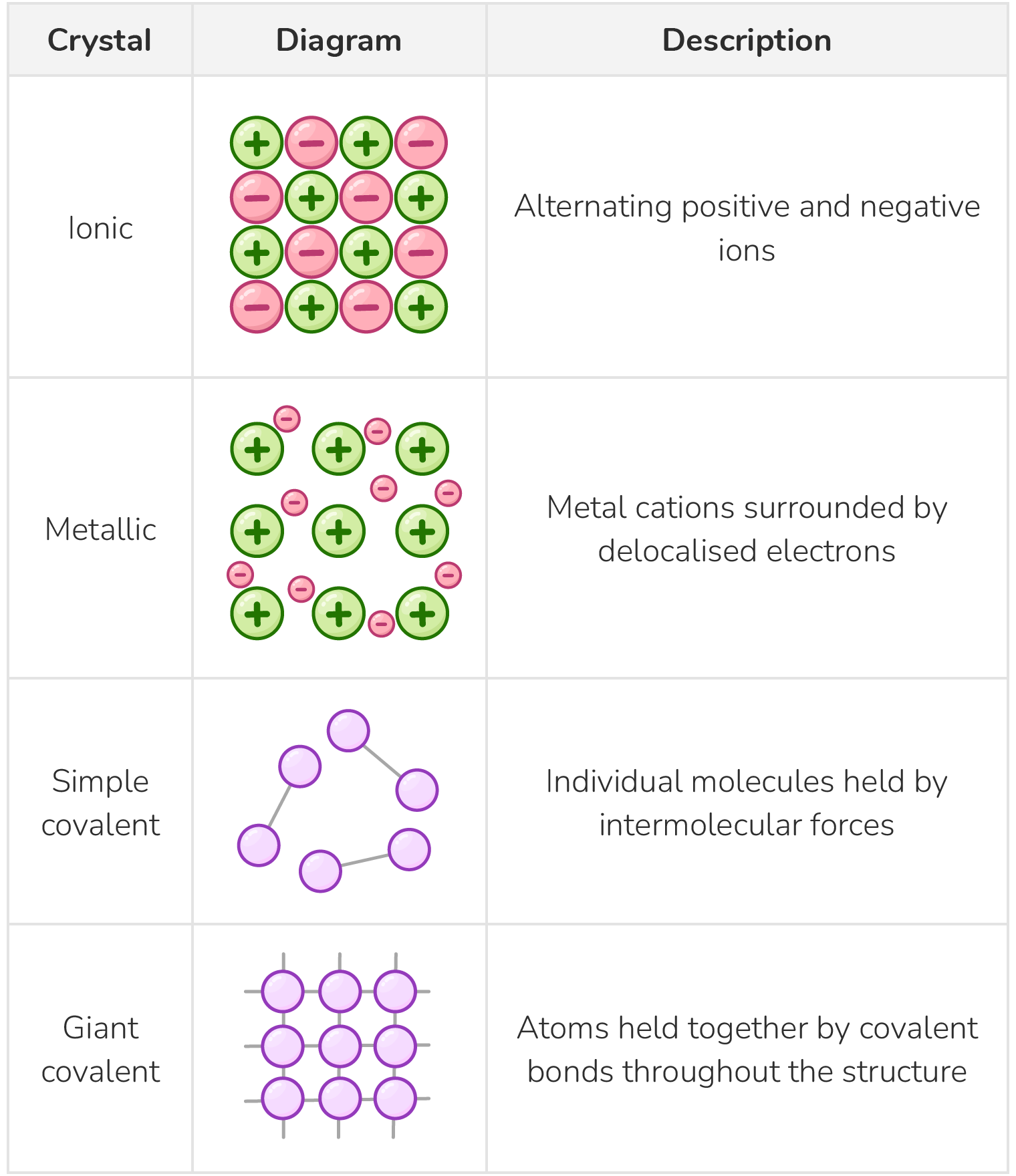
Physical properties of the four crystal types
| Ionic | Metallic | Simple molecular | Giant covalent | |
|---|---|---|---|---|
| Bonding | Ionic | Metallic | Covalent | Covalent |
| Melting & boiling points | High | High | Low | Very high |
| State at room temperature | Solid | Solid | Usually liquid or gas | Solid |
| Electrical conductivity | Conductive when molten or in solution | High conductivity | Non-conductive | Non-conductive (except graphite) |
| Solubility in water | Soluble | Insoluble | Depends on polarity of molecule | Insoluble |
Ionic bonding explains ionic compound properties
High melting and boiling points:
- Strong electrostatic forces between oppositely charged ions require significant energy to overcome.
Electrical conductivity:
- When molten or dissolved, charged ions can move and conduct electricity.
Solubility in water:
- Charged ions interact strongly with polar water molecules, allowing ionic compounds to dissolve.
Metallic bonding explains metal properties
High melting points:
- Strong electrostatic forces between positive ions and delocalised electrons require significant energy to overcome.
Thermal conductivity:
- Delocalised electrons readily conduct thermal energy, making metals good heat conductors.
Electrical conductivity:
- Delocalised electrons can move and carry charge through metals.
Insolubility:
- Metallic bonds are very strong, preventing dissolution.
Simple molecular bonding explains simple molecular properties
Low melting and boiling points:
- Weak intermolecular forces between molecules require little energy to overcome.
Peculiar properties of ice and water due to hydrogen bonding:
- Ice is less dense than water.
- Water has a higher than expected boiling point.
Poor electrical conductivity:
- Absence of charged particles or delocalised electrons prevents electrical conduction.
Solubility depends on molecular polarity:
- Polar molecules (e.g., alcohols) can form hydrogen bonds with water and are soluble.
- Non-polar molecules (e.g., oils) cannot form strong interactions with water and are insoluble.
Giant covalent bonding explains giant covalent properties
Very high melting and boiling points:
- Many strong covalent bonds throughout the structure require significant energy to break.
Electrical conductivity:
- Graphite: Delocalised electrons between carbon layers allow electrical conduction.
- Diamond and silicon(IV) oxide: Localised electrons in four covalent bonds per atom prevent electrical conduction.
Insolubility:
- Strong covalent bonds prevent interaction with water molecules.
Predicting structure from properties
You can use the properties of a material to predict its structure.
Example:
- Substance X has a melting point of 801°C. When solid, it is an insulator, but when molten it conducts electricity well.
- The high melting point and electrical conductivity when molten indicate substance X is ionic. It is not giant covalent because it conducts electricity when molten.
So substance X likely has an ionic crystal lattice structure.