The Mass Spectrometer
This lesson covers:
- How a mass spectrometer works
- Interpreting mass spectra
- Calculating relative atomic mass from mass spectra
- Time-of-flight calculations
How a mass spectrometer works
A mass spectrometer is an analytical tool used to measure the mass-to-charge ratio (m/z) of ions.
The time-of-flight (TOF) mass spectrometer is one commonly used type. It determines the m/z of ions based on how long they take to travel from the ion source to the detector.
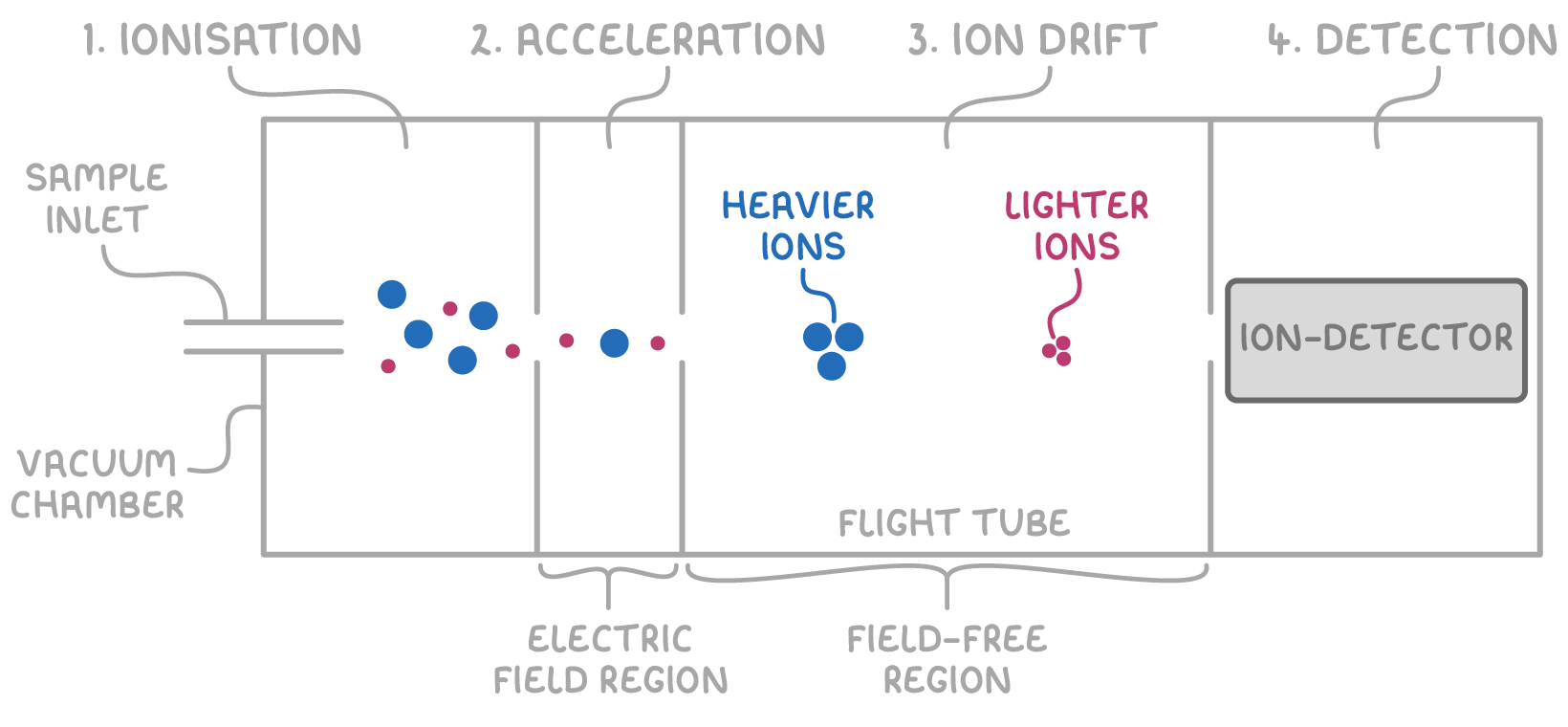
A TOF mass spectrometer includes four main parts:
- Ionisation - This process turns the sample into positive gaseous ions. There are two common methods of ionisation:
- Electron impact (EI) involves bombarding the vaporised sample with high-energy electrons to remove an electron from each molecule, resulting in +1 ions.
- Electrospray ionisation (ESI) involves dissolving the sample in a volatile solvent and spraying it through a charged needle, which adds an H+ ion from the solvent to each molecule.
- Acceleration - The positively charged ions are accelerated towards a negatively charged plate by an electric field, giving them identical kinetic energy. This means lighter ions will move faster than heavier ones.
- Ion drift - The ions enter a flight tube where they drift at a steady speed related to their mass. Lighter ions, having lower m/z ratios, move through the tube quicker and reach the detector sooner than heavier ions.
- Detection - As ions hit the detector, they gain an electron which creates an electric current. The size of this current is proportional to the abundance of each isotope, with more abundant isotopes producing larger currents.
Interpreting mass spectra
A mass spectrum plots the relative abundance of ions against their mass-to-charge ratio (m/z).
- The x-axis displays the m/z values. For electron impact, the m/z of each peak equals the relative mass of the ion.
- The y-axis indicates the relative abundance of each ion, either in arbitrary units or as a percentage.
For elemental samples, each peak represents a different isotope.
- The relative height of each peak shows how abundant the isotope is.
- Elements with only one stable isotope will show a single peak.
Below is the mass spectrum of an element with two stable isotopes.
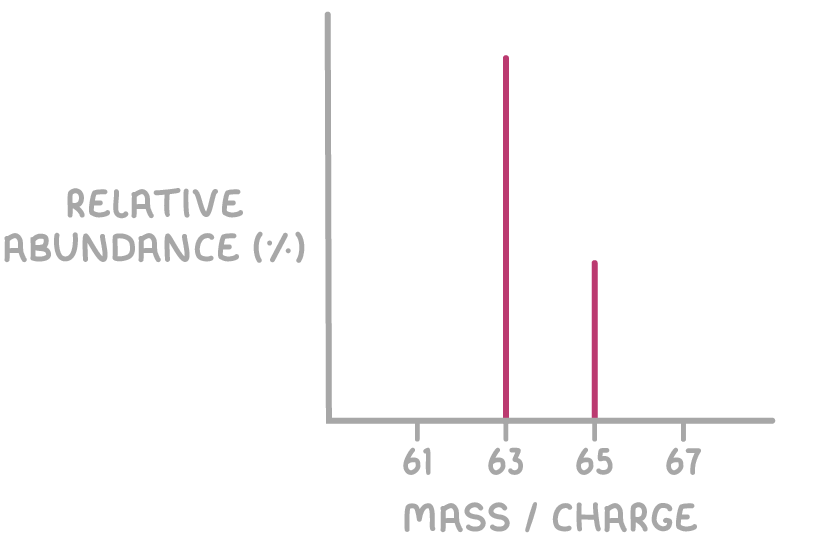
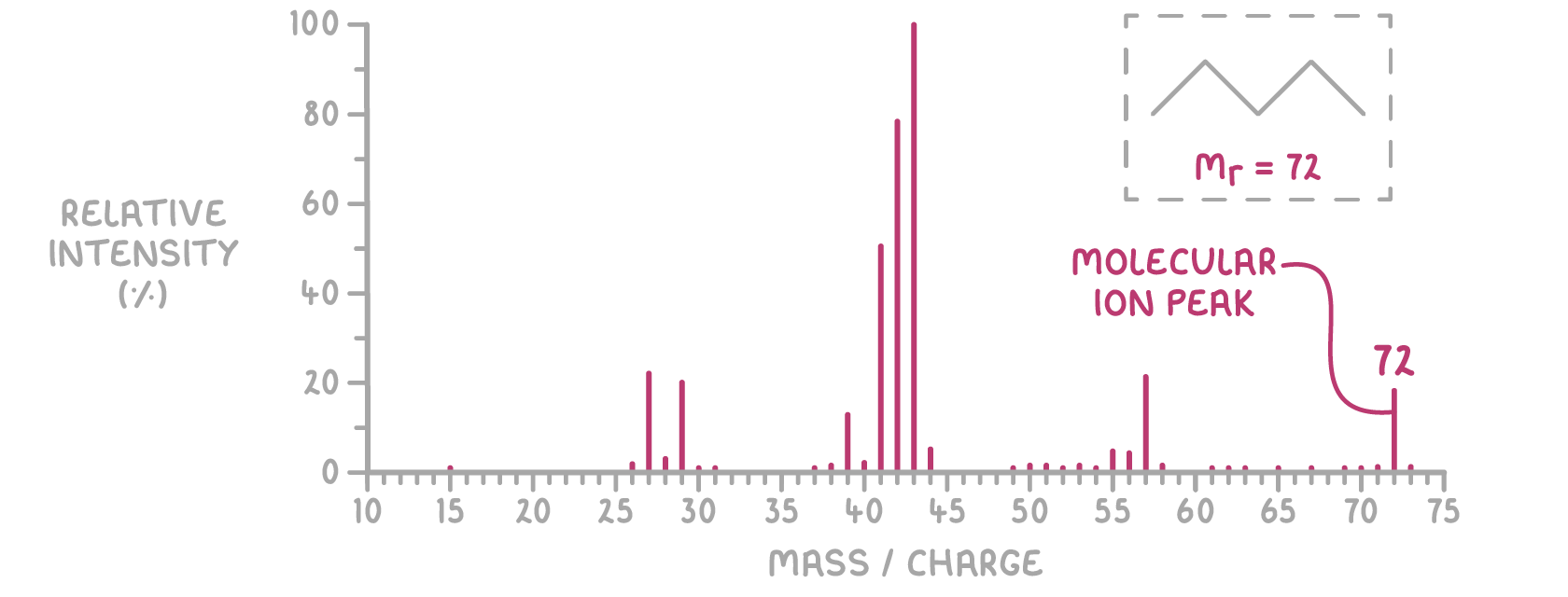
For molecular samples, the peak at the highest m/z value is the molecular ion (M+).
- The m/z of the molecular ion peak matches the molecule's relative molecular mass (Mr).
- Peaks at lower m/z values come from fragments of the molecular ion.
Below is the mass spectrum for a compound with a molecular mass of 72.
Calculating relative atomic mass from mass spectra
To find an element's relative atomic mass (Ar) from its mass spectrum, follow these steps:
- Multiply the relative isotopic mass by its relative abundance for each isotope.
- Add these products together.
- Divide the total by the sum of the relative abundances (100 if using percentages).
Worked example 1 - Calculating the relative atomic mass of copper from its mass spectrum
Calculate the relative atomic mass (Ar) of copper using the mass spectrum shown below:
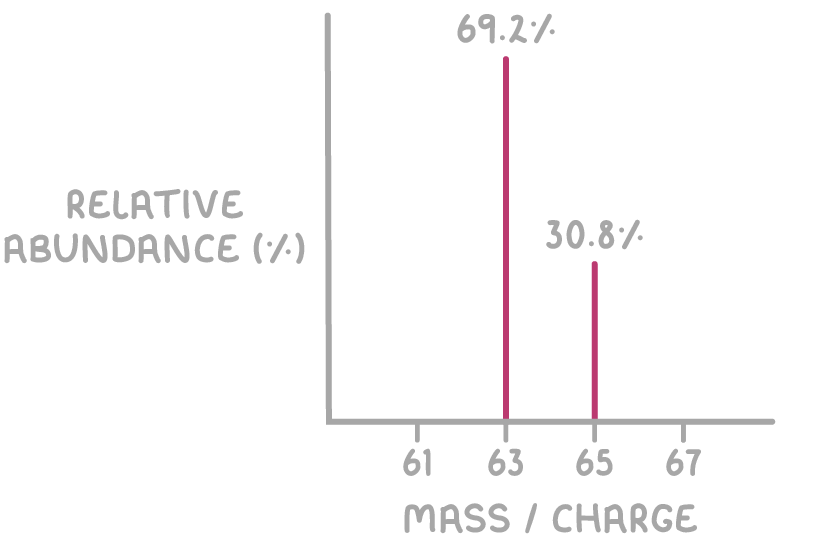
Step 1: Multiply the relative isotopic mass by its relative abundance for each isotope
For 63Cu: 63 x 69.2 = 4,359.6
For 65Cu: 65 x 30.8 = 2,002.0
Step 2: Sum these values
Sum = 4,359.6 + 2,002.0 = 6,361.6
Step 3: Divide by the total relative abundance
Total relative abundance = 69.2 + 30.8 = 100.0
Ar =1006,361.6=63.616
Therefore, the calculated relative atomic mass (Ar) of copper from its mass spectrum is 63.6.
Time-of-flight calculations
In a TOF mass spectrometer, the duration an ion takes to reach the detector is calculated using the flight tube's length and the ion's kinetic energy as it enters the tube.
With all ions having identical kinetic energy as they enter the flight tube, their time of flight (t) is calculable via:
t=d×√2Ekm
Where:
- t = time of flight (s)
- d = length of flight tube (m)
- Ek = kinetic energy (J)
- m = mass of ion (kg)