Formulae of Carbon Compounds
This lesson covers:
- What organic chemistry is
- Different formulae to represent organic compounds
What is organic chemistry?
Organic chemistry is the study of carbon compounds. An organic compound contains carbon bonded to other elements, most commonly hydrogen, oxygen, and nitrogen.
Millions of organic compounds exist because carbon atoms can form four covalent bonds to create carbon chains and rings of many different lengths and shapes. Even small changes to carbon chains can greatly change a compound's properties.
Representing organic compounds
There are several formulae to represent organic compounds:
- General formula - An algebraic formula that describes classes of organic compounds. For example, the general formula for alkanes is CnH2n+2.
- Empirical formula - Expresses the relative numbers of each type of atom in the simplest whole number ratio possible. For example, the empirical formula for butan-1-ol is CH2O.
- Molecular formula - Indicates the actual numbers of atoms of each element in a molecule. For example, the molecular formula of butan-1-ol is C4H10O.
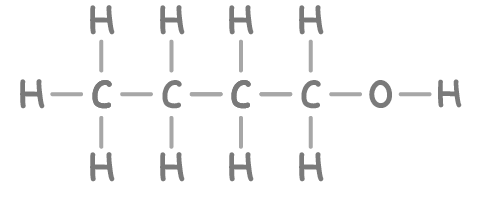
- Structural formula - Illustrates how the atoms are arranged by tracing the carbon backbone, showing which carbons are bonded together. It also indicates which atoms or functional groups are connected to each carbon. For example, the structural formula for butan-1-ol is CH3CH2CH2CH2OH.
- Skeletal formula - Displays the bonding framework of the carbon backbone only with any functional groups. It traces the carbon-carbon bonds, omitting all carbon atoms and attached hydrogen atoms. For example, the skeletal formula of butan-1-ol is shown below.

- Displayed formula - Shows the precise arrangement of all the atoms and all the bonds between them. For example, the displayed formula of butan-1-ol is shown below.