Alkanes
This lesson covers:
- The general formula and bonding of alkanes
- The tetrahedral geometry of alkanes
- How alkane structure affects boiling point
Alkanes are saturated hydrocarbons
Alkanes are a class of hydrocarbons that have the general formula CnH2n+2, where 'n' represents the number of carbon atoms.
Key features of alkanes include:
- They consist solely of carbon and hydrogen atoms (thus called hydrocarbons).
- Each carbon atom forms four single bonds.
- They are fully "saturated" with hydrogen, meaning they contain no double or triple bonds.
Examples of alkanes:
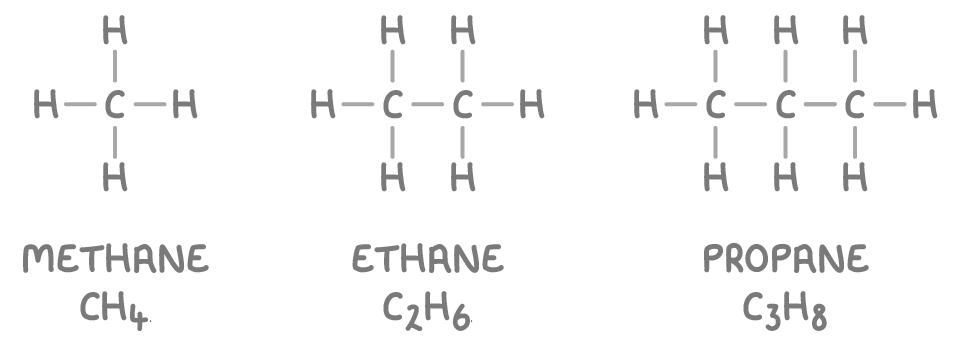
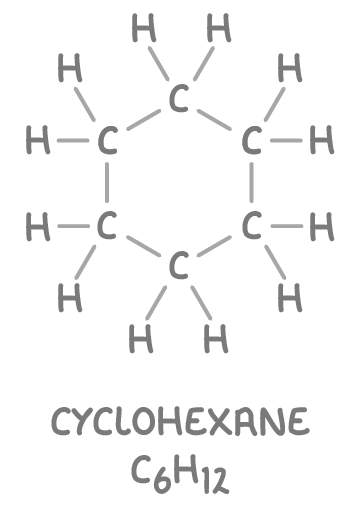
Cycloalkanes are a type of alkane where the carbon atoms form a ring structure. Their general formula is CnH2n, where 'n' is the number of carbon atoms in the ring. Despite the ring structure, cycloalkanes remain saturated.
Tetrahedral geometry of carbon
In alkanes, each carbon atom has:
- Four bonding pairs of electrons.
- These electron pairs are arranged in a tetrahedral geometry because of the equal repulsion between them.
- The bond angles are approximately 109.5°.
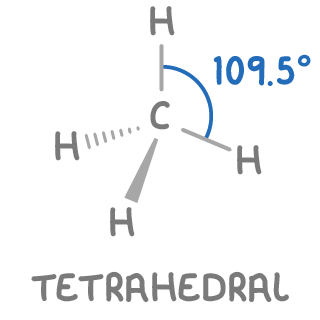
Methane (CH4) is a prime example, forming a perfect tetrahedral shape.
How structure affects boiling points
The boiling point of an alkane is influenced by the strength of its intermolecular induced dipole-dipole forces, which vary based on the length of the carbon chain and the extent of branching.
- Carbon chain length
- A longer carbon chain means more electrons are present, creating stronger temporary inducible dipoles.
- These stronger dipoles result in stronger induced dipole-dipole forces between molecules.
- Consequently, more energy is needed to overcome these forces and boil the alkane.
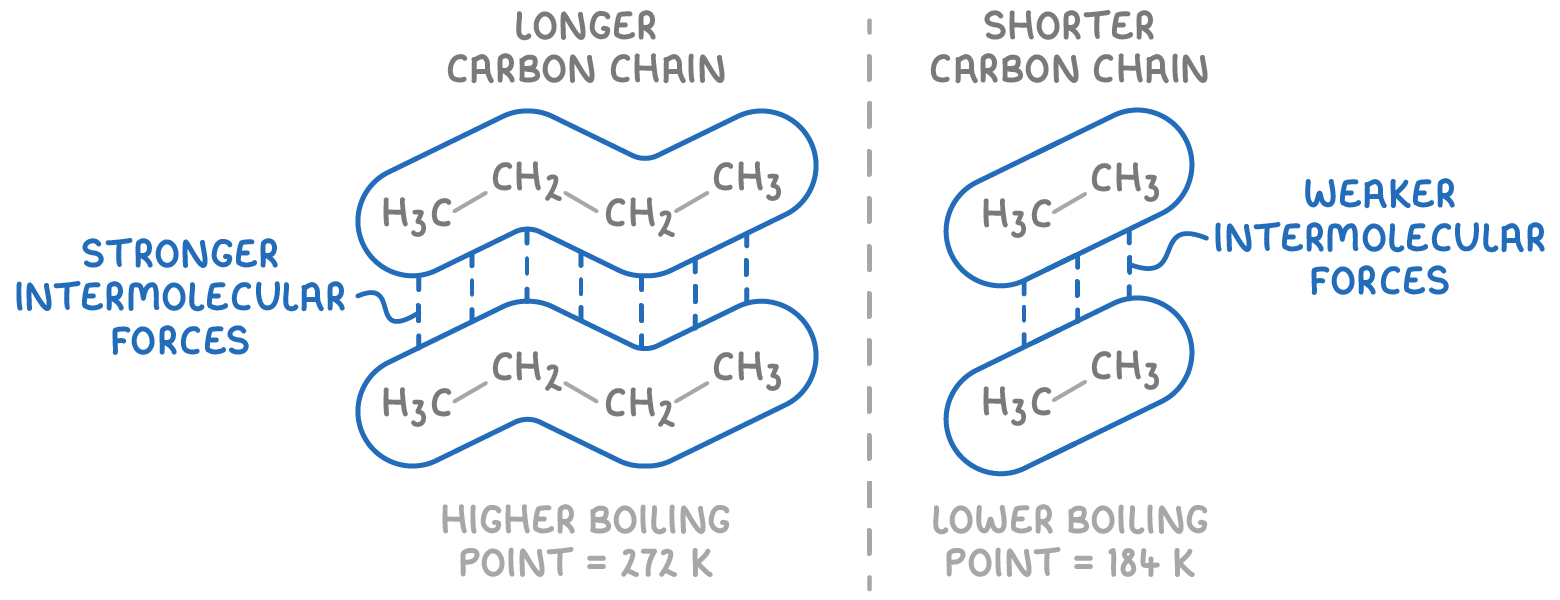
The example above shows that butane (C4H10) has a higher boiling point than ethane (C2H6) due to butane's longer chain which contains more electrons, creating stronger intermolecular forces.
2. Branching:
- Straight chain alkanes can pack together more closely, maximising interaction between their electron clouds. Conversely, branched alkanes have a less efficient packing, reducing electron cloud contact.
- This leads to stronger induced dipole-dipole forces in straight chain alkanes.
- Therefore, more energy is required to separate these molecules.
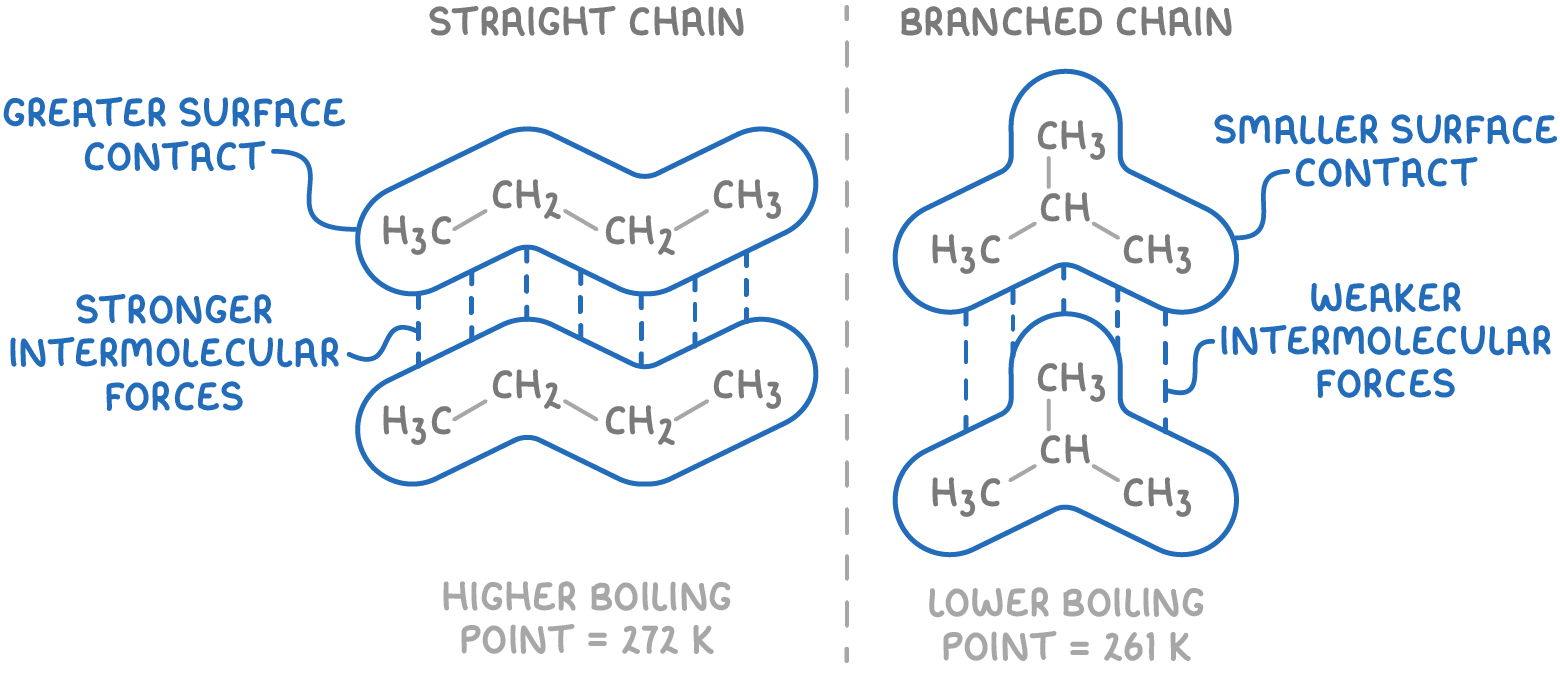
The example above shows that butane has a higher boiling point than its branched isomer, methylpropane due to butane's straight chain which allows greater surface contact between molecules, creating stronger intermolecular forces.