Ligand Substitution Reactions
This lesson covers:
- Ligand substitution reactions and their effect on complex geometry
- Factors affecting complex stability
- The role of Fe2+ complexes in haemoglobin for oxygen transport
- Predicting products of unfamiliar substitution reactions
- Precipitation reactions
Ligand substitution reactions
Ligands bound to a central metal ion in a complex can be exchanged with other ligands in solution. This is called a ligand substitution reaction. This often leads to a colour change in the solution.
If incoming and outgoing ligands are similar in size, the coordination number and geometry do not change:
- [Cr(H2O)6]3+(aq) + 6NH3(aq) ➔ [Cr(NH3)6]3+(aq) + 6H2O(l)
- H2O and NH3 are similar in size, so the chromium ion complex remains octahedral with six coordinated ligands.
- The colour of the solution changes from pale purple to purple.
If ligands differ in size, the coordination number and geometry change:
- [Cu(H2O)6]2+(aq) + 4Cl-(aq) ➔ [CuCl4]2-(aq) + 6H2O(l)
- Cl- ions are much larger than H2O so the copper ion complex changes from 6-coordinate octahedral to 4-coordinate tetrahedral.
- The colour of the solution changes from pale blue to yellow.
Substitution can also be partial:
- [Cu(H2O)6]2+(aq) + 4NH3(aq) ➔ [Cu(NH3)4(H2O)2]2+(aq) + 4H2O(l)
- NH3 partially replaces H2O ligands but the copper ion complex remains octahedral with six coordinated ligands.
- The colour of the solution changes from pale blue to dark blue.
Factors affecting complex stability
Ligand substitution reactions are generally reversible, since the bond strengths of the incoming and outgoing ligands are usually comparable.
However, some factors lead to substantially increased complex stability and essentially irreversible ligand substitutions:
- Ligands that form stronger bonds with the central metal ion
For example, cyanide ions form stronger bonds with iron(III) ions than water molecules:
[Fe(H2O)6]3+(aq) + 6CN-(aq) ➔ [Fe(CN)6]3-(aq) + 6H2O(l)
- Replacement of monodentate ligands with multidentate ligands
Ligand substitution reactions involving the replacement of monodentate ligands with multidentate ligands are essentially irreversible because multidentate ligands form more stable complexes compared to monodentate ligands.
For example, the bidentate ligand ethylenediamine (en) replaces monodentate ammonia ligands:
[Co(NH3)6]2+(aq) + 3en(aq) ➔ [Co(en)3]2+(aq) + 6NH3(aq)
The enhanced stability provided by multidentate ligands is known as the chelate effect and can be explained by the increase in entropy:
- When monodentate ligands get substituted for multidentate ligands, the number of separate particles in solution increases (in the reaction above, the number of particles increases from 4 to 7).
- More particles means higher entropy. Reactions with increased entropy are more thermodynamically favourable.
- Reversing these reactions would decrease entropy, making the formation of multidentate complexes essentially irreversible.
Worked example 1 - Predicting the outcomes of unfamiliar ligand substitutions
A student has a solution of [Co(H2O)6]2+ ions. He adds excess HCl(aq) to a sample of the solution.
Write an equation for this ligand substitution reaction and predict the shape of the complex ion formed.
Step 1: Predict the shape of the complex ion formed
Cl- ligands are larger than H2O ligands. When substitution occurs, the coordination number will decrease from 6 to 4 and the shape of the complex ion will be tetrahedral.
Step 2: Consider the overall charge of the complex ion formed
The H2O ligand has no charge and CN- has a 1- charge, so the overall charge of the complex changes from 2+ to 2-.
Step 3: Write the equation
[Co(H2O)6]2+(aq) + 4Cl-(aq) ➔ [CoCl4]2-(aq) + 6H2O(l)
Worked example 2 - Predicting the outcomes of unfamiliar ligand substitutions
A student has a solution of [Fe(H2O)6]2+ ions. She adds excess ethylenediamine (en)(aq) to a sample of the solution.
Write an equation for this ligand substitution reaction and predict the shape of the complex ion formed.
Step 1: Predict extent of substitution
H2O is a monodentate ligand whereas en is a bidentate ligand. Full substution of H2O with en leads to increased stability of the complex due to the chelate effect. Six H2O ligands are replaced by three en ligands.
Step 2: Predict the shape of the complex ion formed
Substition will occur with no change in coordination number of geometry so the shape of the complex ion formed remains octahedral.
Step 3: Consider the overall charge of the complex ion formed
Since both H2O and en ligands have no charge, the overall charge of the complex remains 2+.
Step 4: Equation
[Fe(H2O)6]2+(aq) + 3en(aq) ➔ [Fe(en)3]2+(aq) + 6H2O(aq)
Haemoglobin oxygen transport
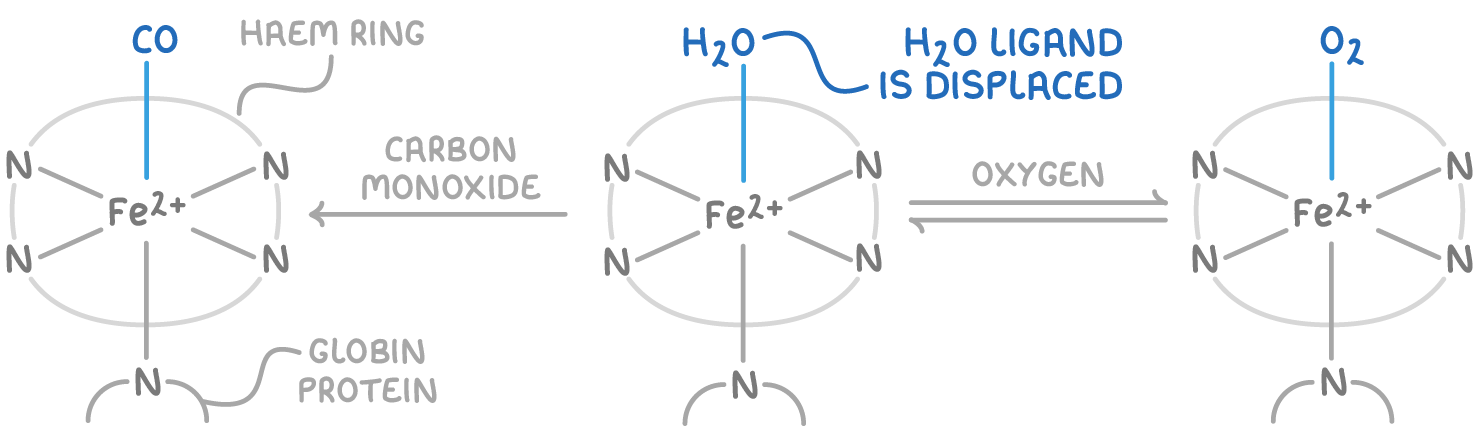
Haemoglobin provides an important example of ligand substitution occurring in the body for oxygen transport:
- Haemoglobin contains Fe2+ ions at its centre. Four nitrogen atoms within a haem molecule provide four ligands to the Fe. A fifth nitrogen from a globin protein provides another ligand. The sixth ligand position starts off occupied by a water molecule.
- In the lungs, where oxygen concentration is high, the water ligand is substituted by an oxygen molecule (O2). This forms oxyhaemoglobin, which travels around the body.
- At body tissues lacking oxygen, the O2 is exchanged back for a water ligand as oxyhaemoglobin releases its oxygen.
- If carbon monoxide (CO) is inhaled, haemoglobin substitutes its water ligand for the CO. This is dangerous because CO binds to the haemoglobin irreversibly, preventing further oxygen transport.
- CO binds irreversibly because it forms a stronger coordination bond with the Fe2+ compared to the O2 ligand.
Precipitates of transition element hydroxides
In aqueous solutions, the transition elements exist as the hydrated ions [M(H2O)6]n+. These can also be written simply as Mn+(aq) where M is the metal.
When aqueous solutions containing transition element ions are mixed with sodium hydroxide (NaOH) or aqueous ammonia (NH3), coloured metal hydroxide precipitates form. In these reactions, the hydroxide or ammonia ligands substitute the water ligands in the metal aqua ions.
The equation for the precipitation of M2+ and M3+ aqua ions with NaOH(aq) is:
- M2+: [M(H2O)6]2+(aq) + 2OH-(aq) ➔ M(H2O)4(OH)2(s) + 2H2O(l)
- M3+: [M(H2O)6]3+(aq) + 3OH-(aq) ➔ M(H2O)3(OH)3(s) + 3H2O(l)
The equation for the precipitation of M2+ and M3+ aqua ions with NH3(aq) is:
- M2+: [M(H2O)6]2+(aq) + 2NH3(aq) ➔ M(H2O)4(OH)2(s) + 2NH4+(aq)
- M3+: [M(H2O)6]3+(aq) + 3NH3(aq) ➔ M(H2O)3(OH)3(s) + 3NH4+(aq)
For some metal ions like Cu2+ and Al3+ excess ammonia or excess sodium hydroxide causes the precipitate to react further to form soluble charged complex ions containing NH3 or OH- ligands.
You need to know the formulae and colours of the precipitates formed when Cu2+, Fe2+, Fe3+ and Al3+ metal aqua ions undergo precipitation reactions with aqueous NaOH and NH3.
Copper(II)
- With NaOH or NH3, a pale blue precipitate of Cu(H2O)4(OH)2 forms from the pale blue aqua ion.
- The precipitate remains insoluble with excess NaOH.
- With excess NH3, a deep blue solution of [Cu(NH3)4(H2O)2]2+ forms:
Cu(H2O)4(OH)2(s) + 4NH3(aq) ➔ [Cu(NH3)4(H2O)2]2+(aq) + 2OH-(aq) + 2H2O(l)
Iron(II)
- With NaOH or NH3, a dark green precipitate of Fe(H2O)4(OH)2 forms from the pale green aqua ion.
- The precipitate remains insoluble with excess NaOH and excess NH3.
Iron(III)
- With NaOH or NH3, a brown precipitate of Fe(H2O)3(OH)3 forms from the yellow aqua ion.
- The precipitate remains insoluble with excess NaOH and excess NH3.
Aluminium
- With NaOH or NH3, a white precipitate of Al(H2O)3(OH)3 forms from the colourless aqua ion.
- With excess NaOH, a colourless solution of [Al(H2O)2(OH)4]- forms:
Al(H2O)3(OH)3(s) + OH-(aq) ➔ [Al(H2O)2(OH)4]-(aq) + H2O(l)
- The precipitate remains insoluble with excess NH3.
Summary of precipitation reactions
The observations of the precipitation reaction of metal aqua ions with OH- and NH3 are summarised in the table below:
| Metal aqua ion | With OH-(aq) or NH3(aq) | With excess OH-(aq) | With excess NH3(aq) |
|---|---|---|---|
| Cu2+ | Blue precipitate of Cu(H2O)4(OH)2 | No change | Deep blue solution of [Cu(NH3)4(H2O)2]2+ |
| Fe2+ | Green* precipitate of Fe(H2O)4(OH)2 | No change | No change |
| Fe3+ | Brown precipitate of Fe(H2O)3(OH)3 | No change | No change |
| Al3+ | White precipitate of Al(H2O)3(OH)3 | Colourless solution of [Al(H2O)2(OH)4]- | No change |