Carbon-13 NMR
This lesson covers:
- How the number of peaks indicates distinct carbon environments
- Using chemical shift data tables to identify carbon environments
- Putting together evidence to determine molecular structures
Number of peaks shows distinct carbon environments
The number of peaks present on a 13C NMR spectrum indicates the number of magnetically distinct carbon environments in the molecule.
Each unique carbon environment produces its own peak - so, a peak represents a set of equivalent carbon atoms.
For example, chloroethane (CH3CH2Cl) has two magnetically different carbon environments and thus two peaks in its 13C NMR spectrum:
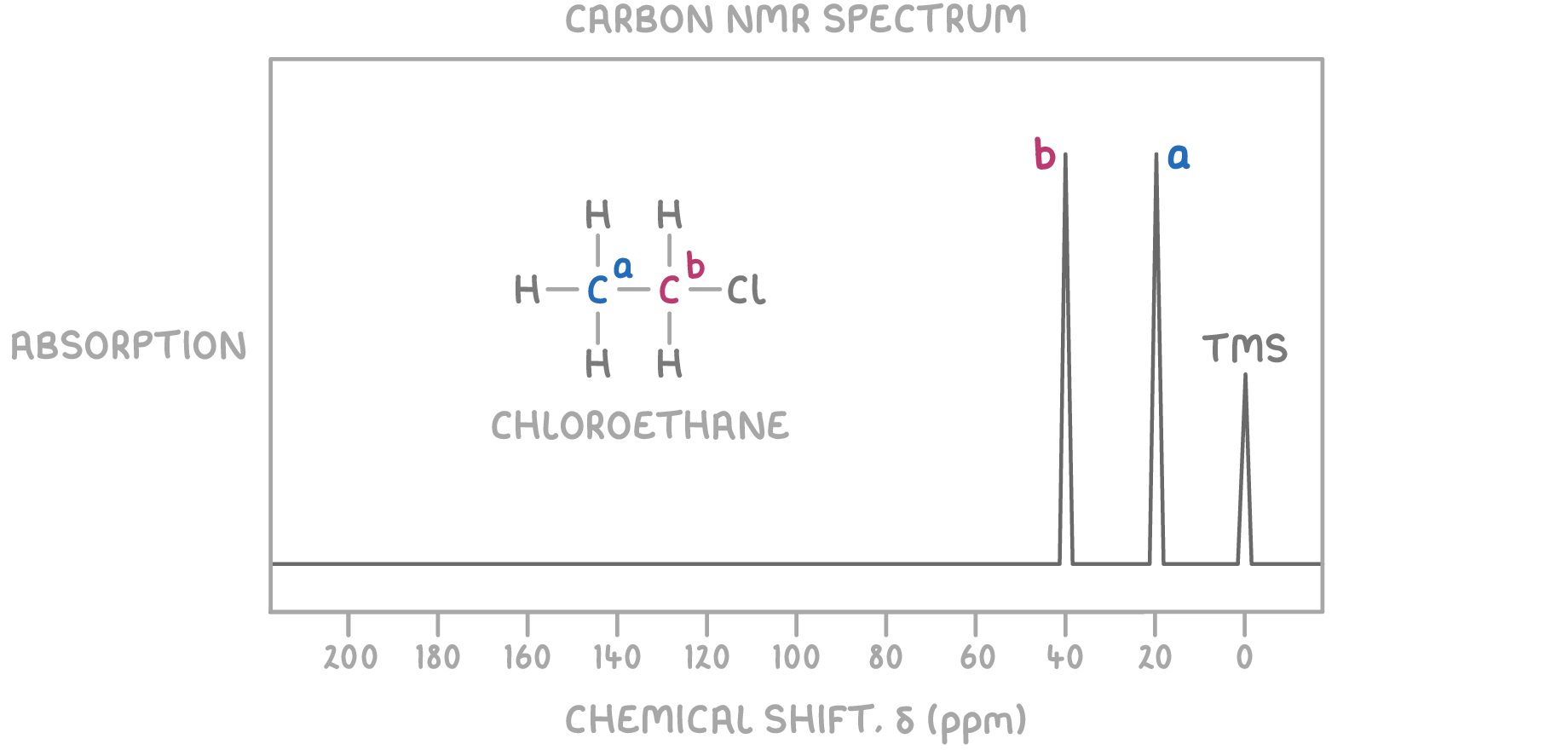
The differing bonding to chlorine versus hydrogen atoms alters the electron density around each carbon, making their environments distinguishable.
Even molecules with multiple carbons, like 1,3-dibromocyclohexane, have a predictable number of peaks based on symmetry:
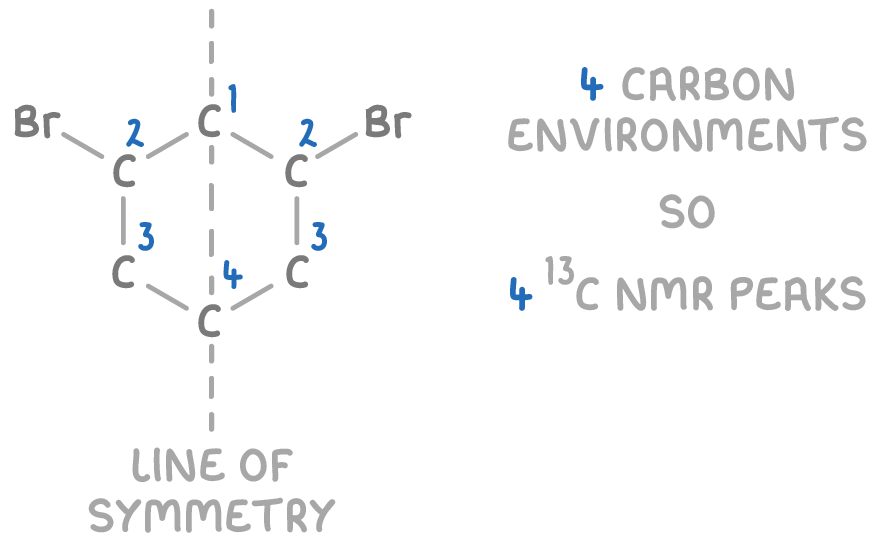
Thus, by carefully examining a molecule’s structure, the number of expected 13C NMR peaks can be determined.
Using chemical shift data tables
Chemical shift values offer insights into the functional groups attached to carbon nuclei.
Data tables compile known chemical shift ranges for different carbon environments relative to TMS:
| Chemical shift, δ (ppm) | Type of carbon |
|---|---|
| 5 – 40 | C–C |
| 10 – 70 | R–C–Cl or R–C–Br |
| 20 – 50 | R–C(=O)–C |
| 25 – 60 | R–C–N (amines) |
| 50 – 90 | C–O (alcohols, ethers or esters) |
| 90 – 150 | C=C (alkenes) |
| 110 – 125 | R–C≡N (nitrile |
| 110 – 160 | aromatic |
| 160 – 185 | R–C=O (ester or carboxylic acid) |
| 190 – 220 | R–C=O (aldehyde or ketone) |
Matching peaks from a spectrum to table entries allows carbon environments to be identified.
However:
- Overlapping shift ranges mean assignments may not be definitive.
- Additional evidence is needed to confirm environment identities.
For example, a peak at ~30 ppm could represent C-C, C-Cl, or C-Br environments based on the table.
Determining molecular structures
Combining evidence from the number of peaks, chemical shift values, and molecular formula allows unknown structures to be deduced.
This methodical approach involves the following steps:
- Count the number of distinct peaks to understand the variety of carbon environments.
- Use the chemical shift data to hypothesise about the types of carbon present.
- Combine all available evidence to propose a molecular structure that fits the given data and the molecular formula.
Worked example 1 - Identifying the structure of an unknown molecule
Using the molecular formula C4H8 and the 13C NMR spectrum provided below, determine the structure of the unknown molecule.
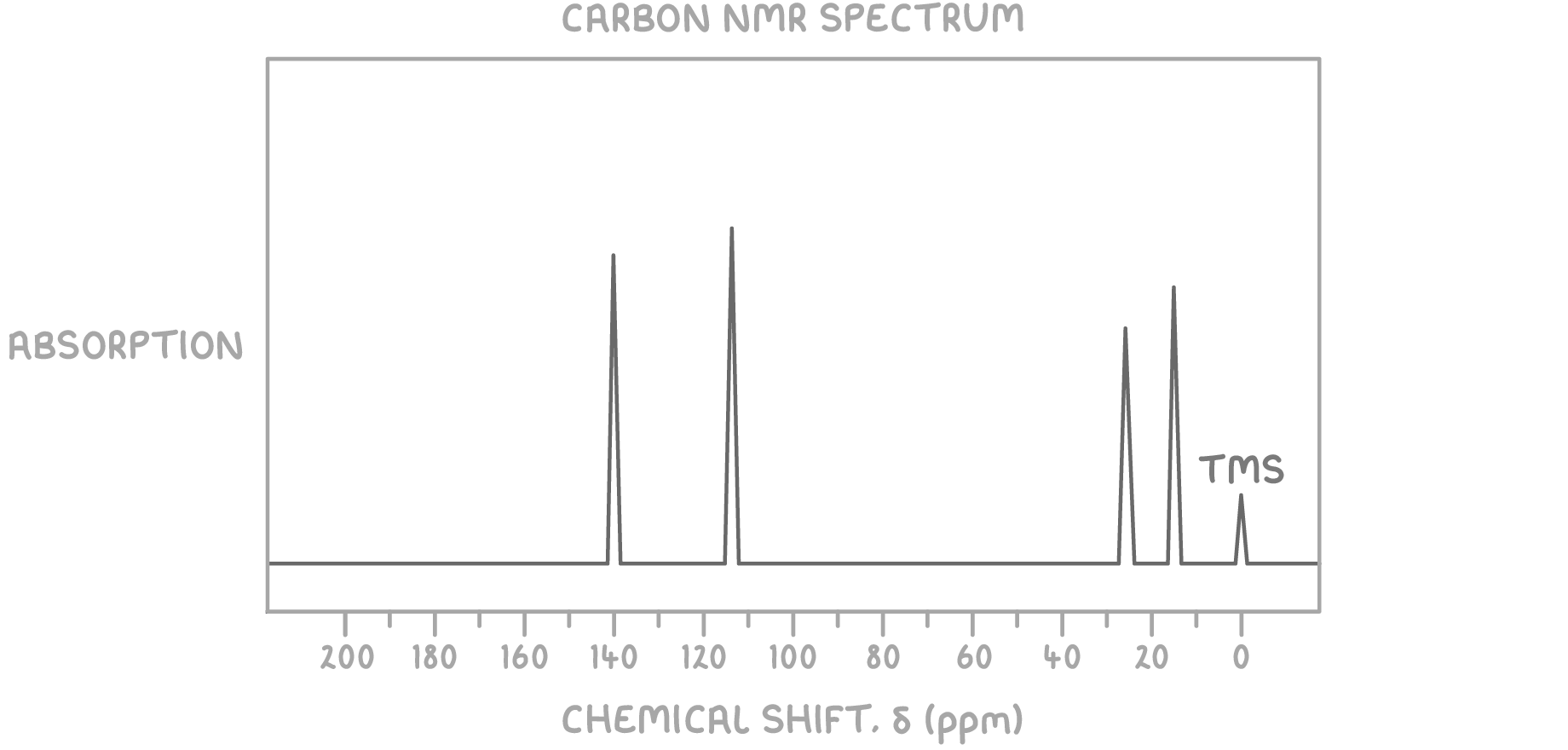
Step 1: Count peaks
The presence of 4 peaks indicates there are 4 magnetically distinct carbon environments in the molecule.
Step 2: Deduce environments from chemical shifts
The peaks at δ 113 ppm and δ 140 ppm suggest carbon atoms involved in a C=C bond, typical for alkenes.
The peaks at δ 13 ppm and δ 27 ppm suggest various types of saturated C-C bond environments, likely representing methyl groups.
Step 3: Consider the molecular formula
Given the molecular formula C4H8, the compound is unsaturated, indicating the presence of a double bond due to the hydrogen count being less than the maximum for a four-carbon alkane (C4H10). This confirms the alkene nature of the compound.
Step 4: Match environments to potential structures
Considering the presence of a double bond indicated by the peaks at δ 113 ppm and δ 140 ppm:
- A straight-chain alkene is required to match the number of carbon peaks observed.
- But-1-ene fits all criteria: it has a double bond creating distinct environments for the carbon atoms (at δ 113 ppm and δ 140 ppm), and two distinct types of C-C environments corresponding to the peaks at δ 13 ppm and δ 27 ppm.
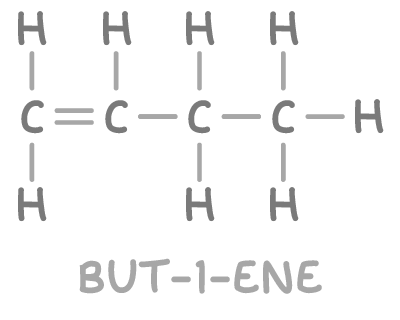
Therefore, the unknown molecule is identified as but-1-ene.
Worked example 2 - Identifying the structure of an unknown molecule
Using the molecular formula C4H10O and the 13C NMR spectrum provided below, determine the structure of the unknown molecule.
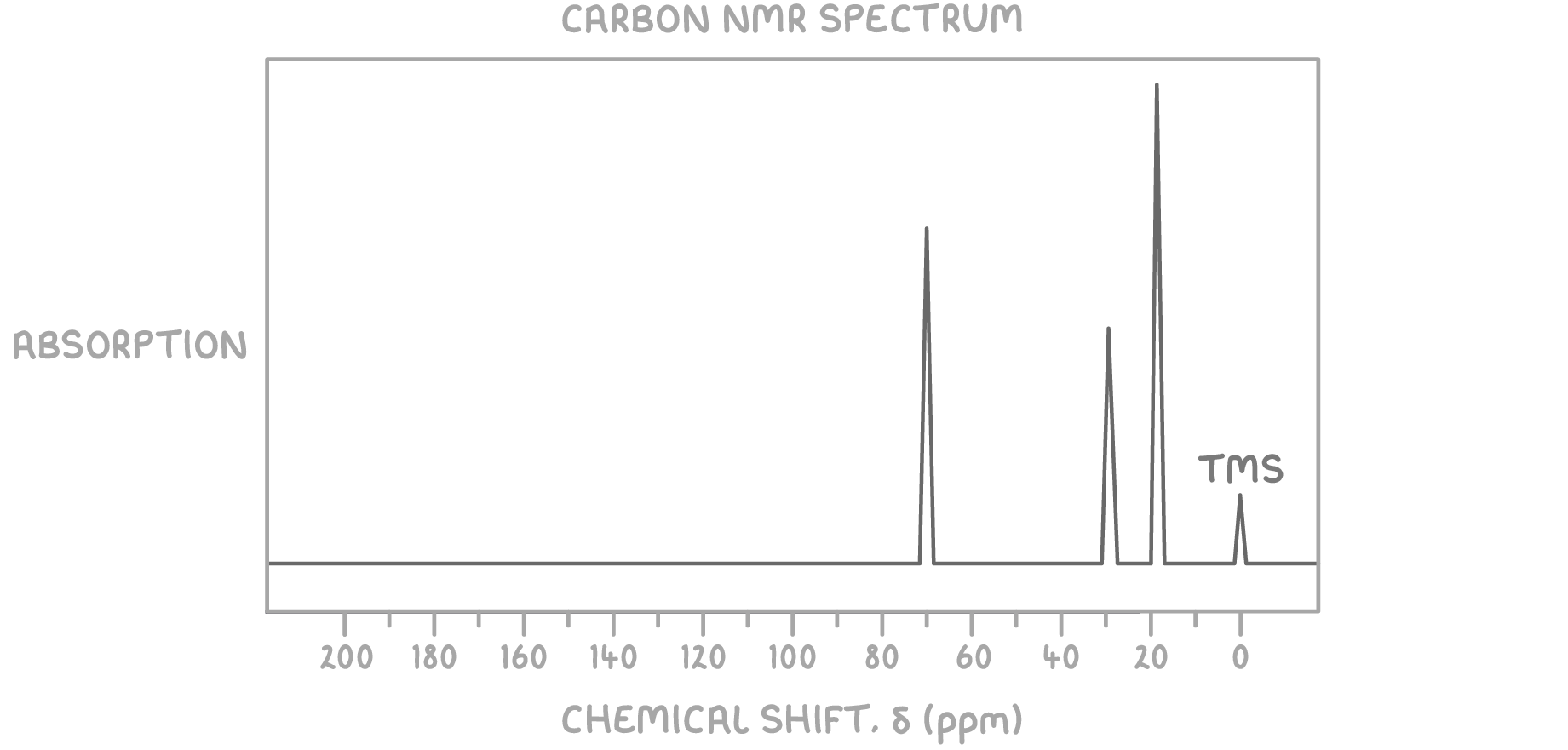
Step 1: Count peaks
The presence of 3 peaks indicates there are 3 magnetically distinct carbon environments in the molecule.
Step 2: Deduce environments from chemical shifts
The peak at δ 70 ppm suggests a C-O bond, typical for alcohols.
The peaks at δ 19 ppm, and δ 31 ppm suggest various types of C-C bond environments, indicating a chain with branching or differing degrees of saturation.
Step 3: Consider the molecular formula
Given the molecular formula C4H10O, the compound is saturated, indicating the absence of double or triple bonds. The presence of oxygen and the saturation level suggest an alcohol.
Step 4: Match environments to potential structures
Considering the alcohol functional group suggested by the δ 70 ppm peak:
- A straight-chain alcohol would not adequately account for the diversity of the chemical shift values observed.
- A molecule like 2-methylpropan-1-ol fits all criteria: it has an C-OH alcohol group at δ 70 ppm (carbon c) and two distinct types of C-C environments corresponding to the peaks at δ 19 ppm and δ 31 ppm.
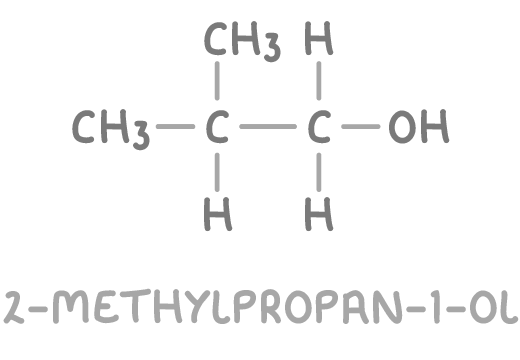
Therefore, the unknown molecule is identified as 2-methylpropan-1-ol.