Collision Theory
This lesson covers:
- Defining reaction rate
- Measuring reaction rate
- Calculating reaction rate from graphs
- Collision theory and activation energy
- The factors that affect rate of reaction
Reaction rate
Reaction rate is defined as the change in concentration (or amount) of a reactant or product over time.
A simple formula for finding the rate of a chemical reaction is:
Measuring reaction rates
You can follow the rate of a reaction by measuring either how fast the reactants are used up or how fast the products are formed. Here are three common methods:
- Measuring a decrease in mass
- For reactions that produce gases, you can measure the rate by observing the decrease in mass over time.
- As gas is released, the mass decreases. This method is straightforward but should be conducted in a fume cupboard for safety.
- Measuring the volume of gas given off
- Use a gas syringe to measure the gas volume produced over time.
- This method is accurate for reactions that generate gases.
- Timing how long a precipitate takes to form (see below)
- This approach is suitable when the product of the reaction is a precipitate.
- Measure how long it takes for a mark to become obscured by the precipitate.
- For consistent comparison of reaction rates, always use the same observer and reference mark.
Calculating rates from graphs
Plotting the amount of reactant used or product formed against time on a graph, the slope (or gradient) of the curve represents the reaction rate.
To calculate the gradient of the graph:
Where:
- y = amount of reactant or product
- x = time
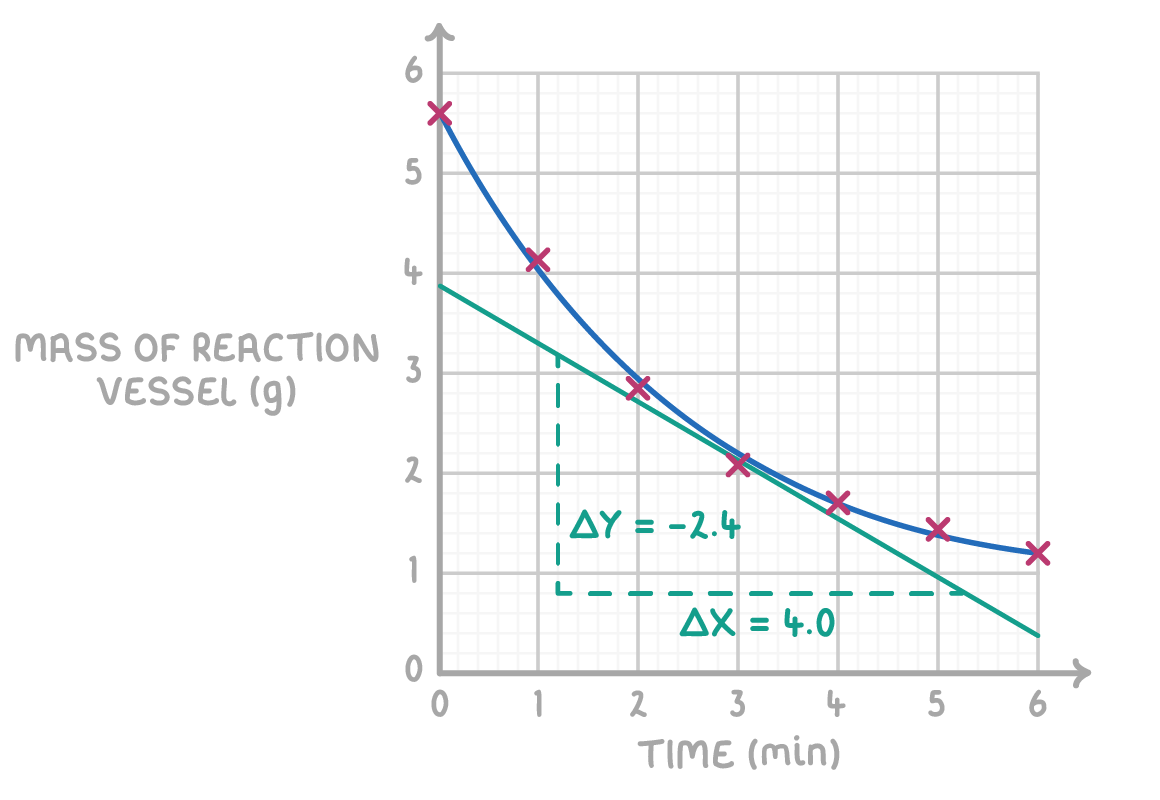
Worked example 1 - Calculating average rate of reaction
The graph below shows the mass of a reaction vessel measured at regular intervals during a chemical reaction.
Calculate the average rate of reaction.
Step 1: Draw a line of best fit
Draw a line of best fit through the data points, starting from the origin (0,0)
Step 2: Choose two distinct points on the line
Identify and choose two points where the line of best fit intersects a gridline distinctly.
Step 3: Create a triangle
Connect these points using vertical and horizontal lines to form a right-angled triangle.
Step 4: Gradient equation
Gradient =ΔxΔy
Step 5: Substitution and correct evaluation
Gradient =4.8−1.24.0−1.0=3.63.0=0.83 cm3 min−1
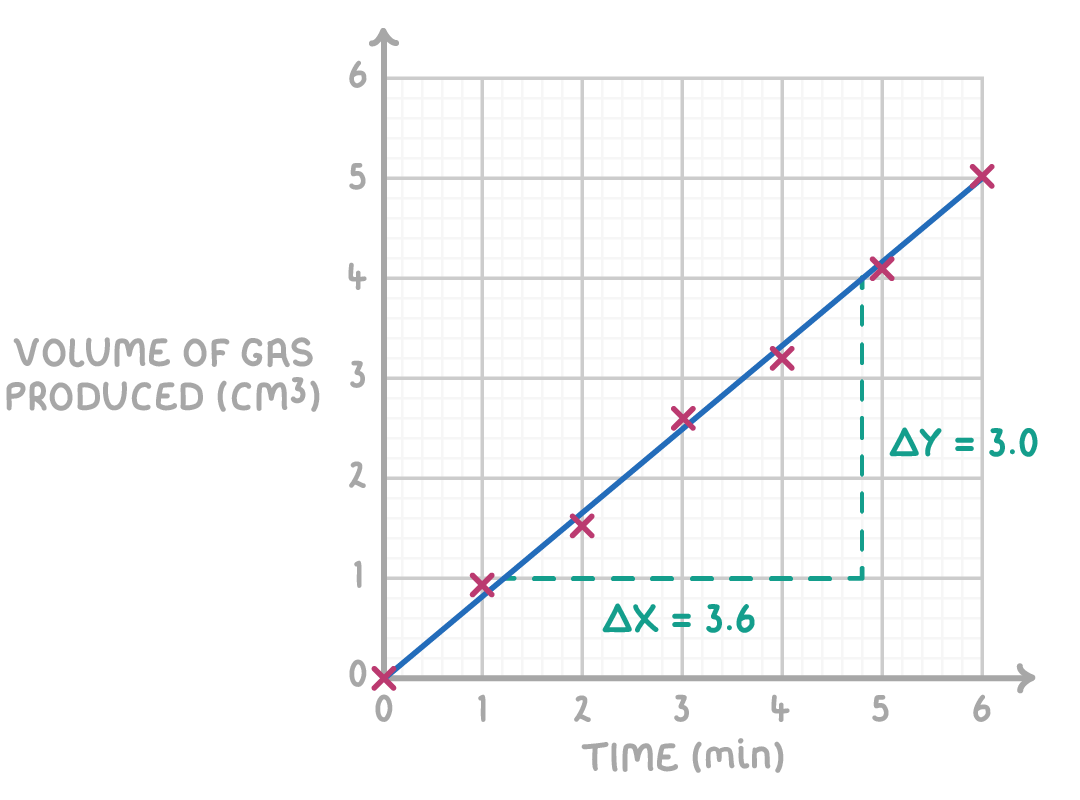
Worked example 2 - Calculating rate of reaction at a specific time
The graph below shows the mass of a reaction vessel measured at regular intervals during a chemical reaction.
Calculate the rate of reaction at 3 minutes.
Step 1: Draw a tangent at 3 minutes
Use a ruler to carefully draw a tangent to the curve at the 3 minute mark on the graph. Extend the tangent across the graph.
Step 2: Choose two distinct points on the tangent
Identify and choose two points where the tangent intersects a gridline distinctly.
Step 3: Create a triangle
Connect these points using vertical and horizontal lines to form a right-angled triangle.
Step 4: Gradient equation
Gradient =ΔxΔy
Step 5: Substitution and correct evaluation
Gradient =5.2−1.20.8−3.2=4.0−2.4=−0.60 g min−1
Collision theory and activation energy
Collision theory states that for a reaction to occur between particles, two conditions must be met:
- Orientation - The particles must collide in the correct orientation. They need to be facing each other appropriately.
- Energy - The colliding particles need at least a minimum amount of kinetic energy. This minimum energy is called the activation energy (Ea).
For a collision to be effective and lead to a reaction, it must meet both the orientation and energy requirements. However, most collisions are non-effective because they fail to satisfy one or both conditions. As a result, these collisions do not lead to a reaction.
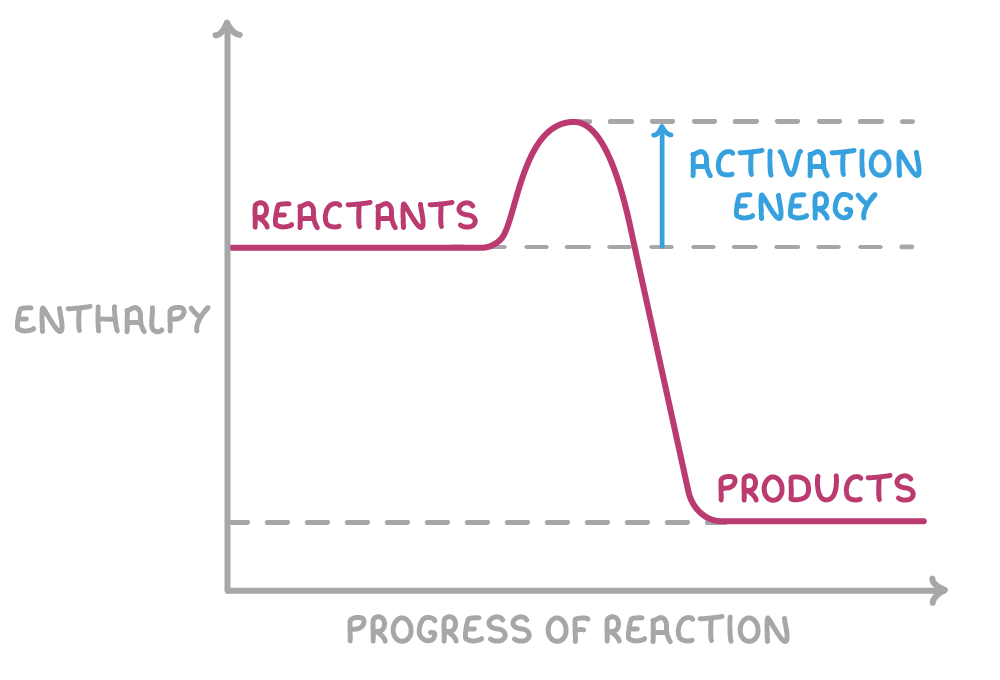
The activation energy, represented in the enthalpy profile diagram above, is the minimum energy required for a collision to be effective. This energy is necessary to break existing bonds in the reactants and initiate the reaction. Particles with kinetic energies greater than or equal to the activation energy will have sufficient energy to react upon collision.
Reactions with low activation energies tend to occur more readily, while those with high activation energies occur less easily. Adding heat energy to the particles can provide the extra energy needed for reactions with high activation energies to proceed.
Factors affecting rate of reaction
The 5 key factors that affect the rate of a chemical reaction are:
- Surface area (of solids)
- Concentration (of solutions)
- Pressure (of gases)
- Temperature
- Catalyst
We can use collision theory to explain why each of these factors impacts reaction rate by considering their effect on the number of effective collisions.
Increasing surface area increases rate of reaction
- When the exposed surface area of the solid is increased, more particles on the surface are available to collide and react. This leads to a higher frequency of effective collisions between the solid and other reactants.
- For example, crushing a solid into a powder provides more exposed surface.
- Therefore, increasing the surface area of a solid reactant results in an increased reaction rate.
Increasing concentration increases rate of reaction
- If the concentration of reactants in solution is increased, the particles will on average be closer together.
- Particles that are closer together will collide more frequently, increasing the number of effective collisions.
- Therefore, increasing the concentration increases the reaction rate.
Increasing pressure increases rate of reaction
- Raising the pressure forces the gas particles closer together.
- Particles that are closer together will collide more frequently, increasing the number of effective collisions.
- Therefore, increasing the pressure increases the reaction rate.
Increasing temperature increases rate of reaction
- Raising the temperature increases the kinetic energy and speed of the particles.
- As the particles move faster, they collide more frequently, resulting in an increased frequency of collisions.
- Additionally, the increased kinetic energy that more particles have the necessary energy to overcome the activation energy, resulting in a greater proportion of effective collisions.
- Therefore, increasing temperature results in an increased reaction rate.
Adding a catalyst increases rate of reaction
- A catalyst provides an alternative pathway or mechanism for the reaction that has a lower activation energy.
- A lower activation energy means particles require less kinetic energy to react.
- More particles have the activation energy, leading to an increased number of effective collisions.
- Consequently, adding a catalyst increases the overall rate of reaction.