DNA
This lesson covers:
- DNA as a polymer of nucleotides
- How DNA nucleotides join to form the sugar-phosphate backbone
- The double helix structure of DNA
- Complementary base pairing in DNA
DNA is a polymer made of nucleotides
DNA, or deoxyribonucleic acid, is the essential molecule that carries genetic information in living beings. It is composed of monomers called nucleotides.
Each nucleotide is made up of three parts:
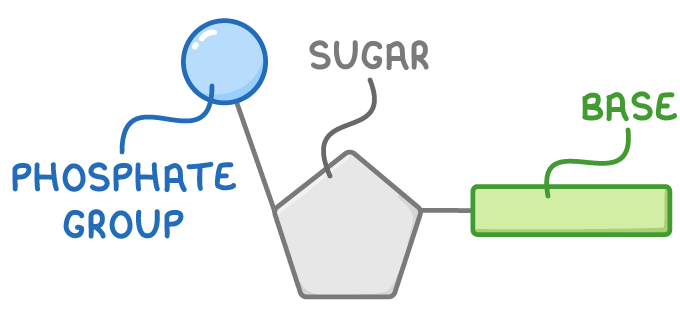
- A phosphate group
- A pentose sugar - This is 2-deoxyribose in DNA
- One of four nitrogenous bases - These are adenine (A), thymine (T), cytosine (C), or guanine (G).
Note: For your exams, the structures of these nucleotides are provided, so you don't need to memorise them.
Nucleotides join through phosphodiester bonds
Nucleotides join together through phosphodiester bonds. These are covalent bonds that form between the phosphate group of one nucleotide and the sugar group of another nucleotide. The repetition of these phosphodiester bonds between successive nucleotides creates the sugar-phosphate backbone of the DNA molecule, a section of which is shown below.
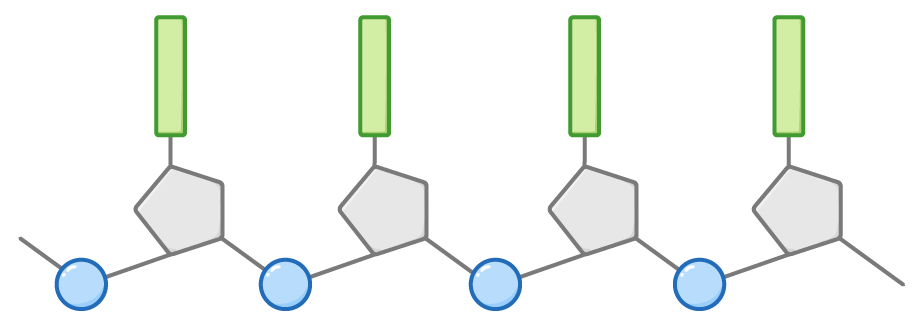
The nucleotides are linked via condensation reactions that form phosphodiester bonds, with the release of a water molecule each time a nucleotide is added to the growing chain.
This condensation reaction between two nucleotides, forming the phosphodiester bond that joins them, is illustrated below.
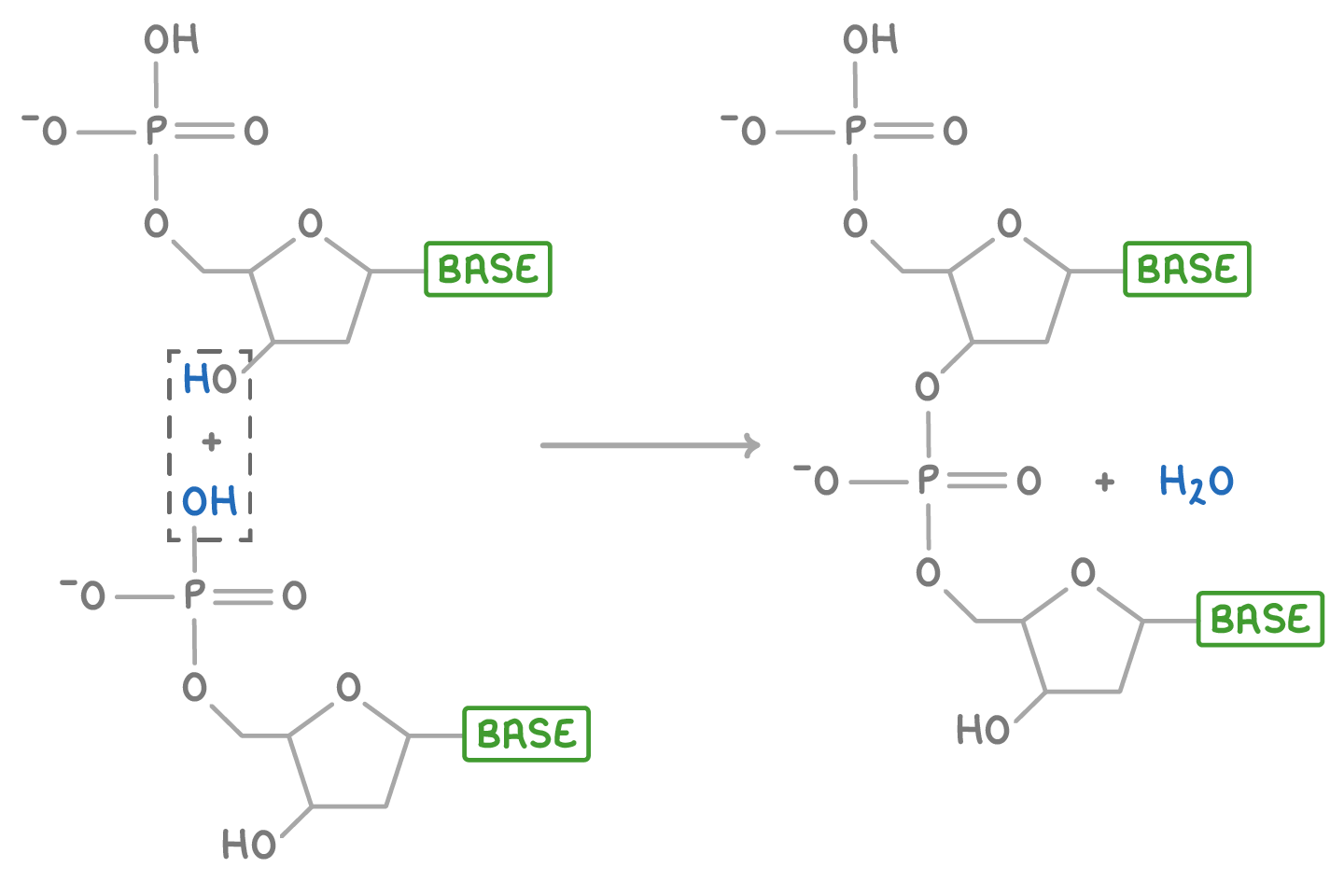
DNA structure is a double helix
The structure of DNA is characterised by two main features:
- It is made of two polynucleotide strands that run in opposite directions, known as antiparallel.
- These strands twist around each other to form a shape known as a double helix.
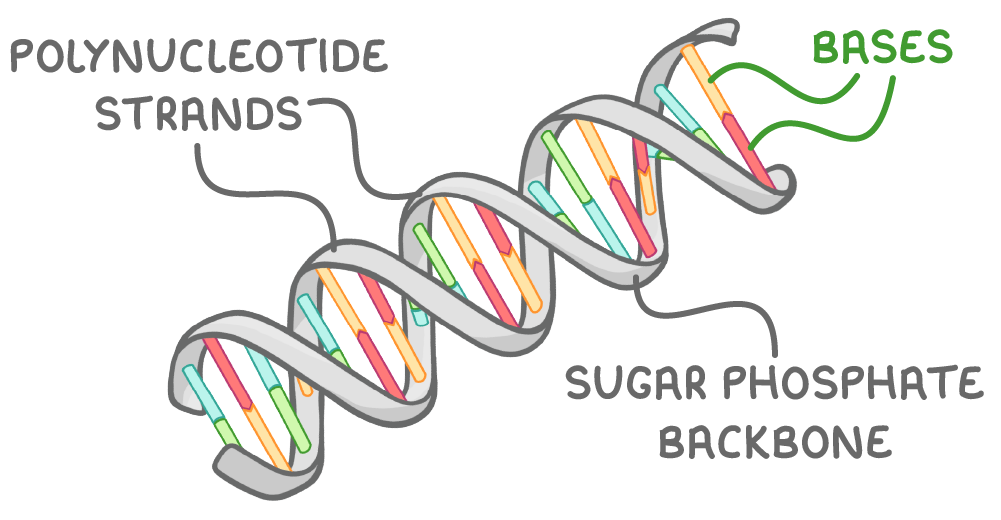
The strands are held together by hydrogen bonds between pairs of bases that complement each other: adenine (A) pairs with thymine (T), and cytosine (C) pairs with guanine (G).
Specific base pairing depends on hydrogen bonds
The specific pairing of bases is due to hydrogen bonding:
- Adenine and thymine form two hydrogen bonds with each other.
- Cytosine and guanine form three hydrogen bonds with each other.
The arrangement of atoms in each base allows for hydrogen bonds to form only between specific pairs, ensuring that A always pairs with T, and C with G. This specificity is critical for the double helix structure, as it allows the bases to be precisely aligned for optimal hydrogen bonding. This intricate molecular recognition ensures the stability and integrity of DNA's double helix structure.