Reactions of carboxylic acids
With carbonates:
- Carboxylic acids react with carbonates to form a carboxylate salt, carbon dioxide and water.
- The carbon dioxide rapidly evolves as bubbles when the reaction occurs. This observable effervescence serves as a simple test to confirm the presence of a carboxylic acid.
- For example, ethanoic acid reacts with sodium carbonate to form sodium ethanoate, carbon dioxide and water:
2CH3COOH(aq) + Na2CO3(s) ➔ 2CH3COONa(aq) + CO2(g) + H2O(l)
With bases:
- Carboxylic acids are neutralised by bases such as metal oxides and hydroxides to form carboxylate salts and water.
- For example, ethanoic acid reacts with sodium hydroxide to form sodium ethanoate and water:
CH3COOH(aq) + NaOH(aq) ➔ CH3COONa(aq) + H2O(l)
Esterification
- Esters, characterised by the -COO- functional group, are made by heating carboxylic acids with alcohols in the presence of concentrated H2SO4 catalyst.
- For example, ethanoic acid reacts with ethanol to form the ester ethyl ethanoate and water:
CH3COOH(aq) + CH3CH2OH(aq) ⇌ CH3COOCH2CH3(aq) + H2O(l)
Forming carboxylic acids
Carboxylic acids can be synthesised through various methods:
- Oxidation of primary alcohols or aldehydes with oxidising agents such as acidified potassium dichromate (K2Cr2O7) or acidified potassium dichromate (KMnO4).
The general reactions are: RCH2OH + [O] ➔ RCHO + H2O + [O]➔ RCOOH
- Hydrolysis of esters involves refluxing esters with either dilute hydrochloric acid (HCl), water, or aqueous sodium hydroxide (NaOH).
The general reaction is: RCOOR' + H2O ⇌ RCOOH + R'OH
Carboxylic acids form hydrogen bonds
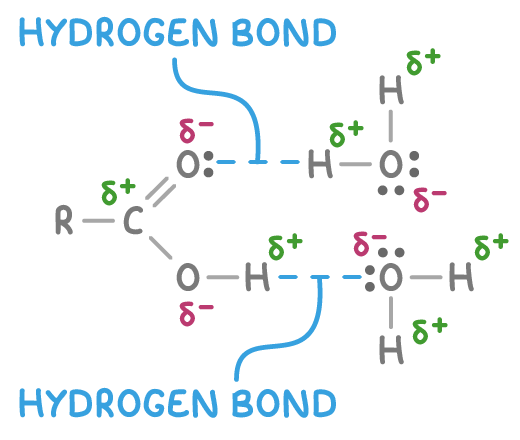
Carboxylic acids are polar due to the electronegative oxygen atoms drawing electrons away from the hydrogen atoms.
This polarity allows carboxylic acids to readily form hydrogen bonds with each other and with water molecules.
Hydrogen bonding makes small carboxylic acids very soluble in water, as they can hydrogen bond with water molecules.
Carboxylic acids are weak acids
In an aqueous solution, carboxylic acids partially dissociate into hydrogen (H+) ions and carboxylate (RCOO-) ions.
For example, ethanoic acid partially dissociates in water:
CH3COOH + H2O ⇌ CH3COO- + H+
This equilibrium lies to the left as most molecules do not dissociate - this makes carboxylic acids weak acids.
Carboxylic acids contain -COOH group
Carboxylic acids contain the carboxyl functional group -COOH. To name them, find the longest carbon chain, remove the 'e' from the alkane name, and add '-oic acid'.
Some examples of carboxylic acids are:
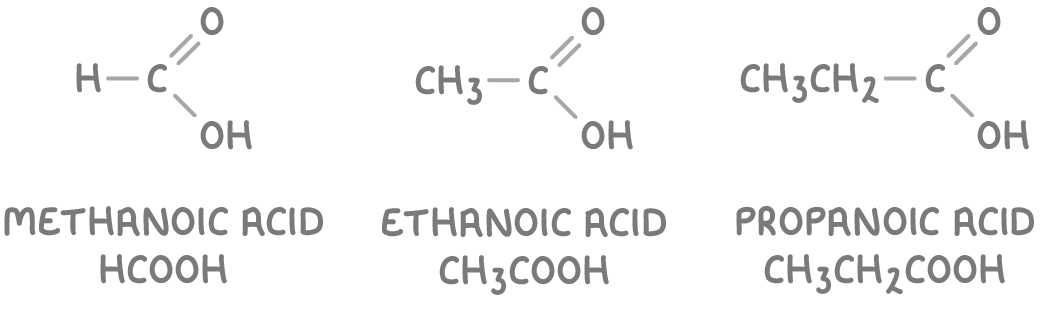
The -COOH group is positioned at the end of the carbon chain and is given priority in naming over other functional groups.
Carboxlic Acids
This lesson covers:
- The structure and naming of carboxylic acids
- Properties of carboxylic acids
- Synthesis of carboxylic acids
- Reactions of carboxylic acids