Addition Polymers
This lesson covers:
- How alkenes can polymerise to form addition polymers
- Drawing and naming addition polymers
- The properties of addition polymers
Alkenes polymerise by opening their double bonds
Alkenes contain a carbon-carbon double bond.
This bond can open up, allowing alkene molecules to join end-to-end and form long chains called polymers.
- The individual alkene molecules are called monomers.
- Joining many monomers together is called polymerisation.
- Polymers made this way are called addition polymers because the double bond opens up and monomer units are added to the chain.
For example, ethene monomers polymerise to form the addition polymer poly(ethene):
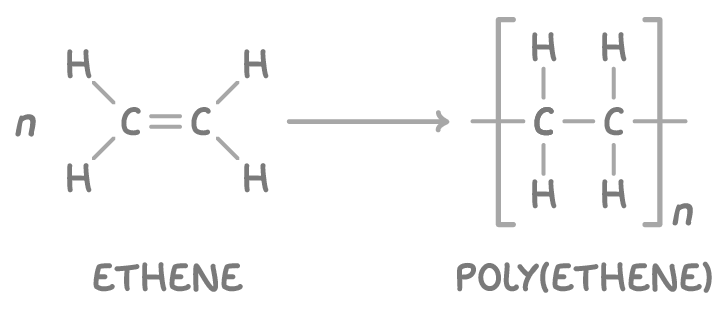
Drawing polymer repeating units
Polymers are made up of smaller repeating units. A repeating unit is the smallest section of a polymer chain that repeats over and over.
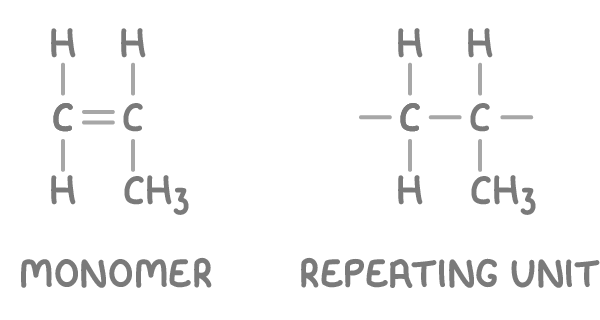
To draw the repeating unit from given monomer:
- Replace the carbon-carbon double bond with a single bond.
- Extend single bonds from each carbon atom to represent attachment sites within the polymer chain.
For example, the repeating unit of poly(propene) can be deduced from the propene monomer.
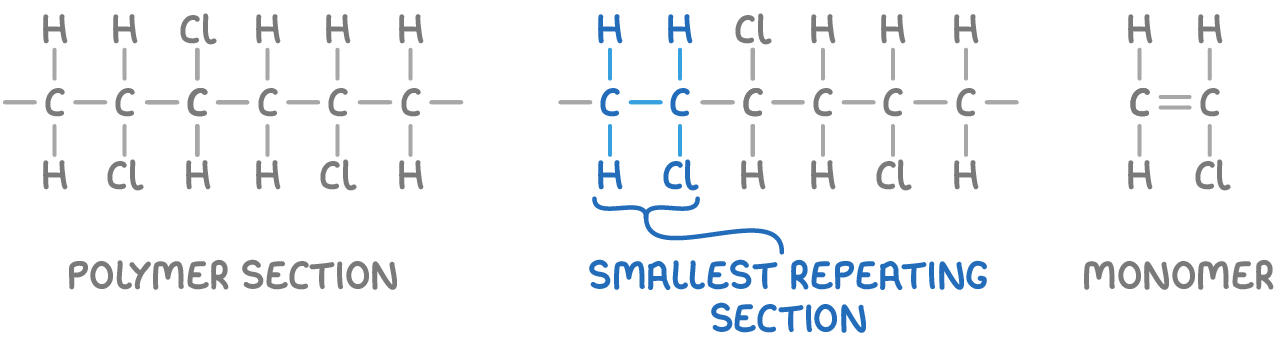
To deduce the monomer from a given polymer section:
- Identify the smallest section that repeats in the full polymer chain.
- Replace the carbon-carbon single bond with a double bond.
For example, the monomer of poly(chloroethene) can be deduced from a section of the polymer chain.
Naming addition polymers
Addition polymers made from alkenes follow systematic naming rules:
- Take the name of the alkene monomer.
- Enclose the monomer name in brackets.
- Add the prefix "poly".
For example, the addition polymer made from butene is poly(butene).
Addition polymers made from alkenes are called polyalkenes.
Physical properties of polyalkenes depend on intermolecular forces
The physical properties of polyalkenes are influenced by the strength of intermolecular forces between the polymer chains:
- Chain length - Longer chains allow more surface contact between neighbouring chains. This strengthens the intermolecular forces.
- Branching - Straight chains can pack together closely in an ordered structure, maximising intermolecular forces. Highly branched chains cannot pack as efficiently, reducing contact between chains.
Longer, straight polyalkenes experience stronger intermolecular forces which requires more thermal energy to overcome. This makes these polymers harder and more rigid. Shorter or branched polyalkenes have weaker intermolecular forces, making them more flexible materials.
Plasticisers modify polymer properties
Plasticisers are small molecules that can be added to polymers to increase flexibility:
- Plasticiser molecules position themselves between polymer chains.
- This pushes the chains apart, weakening intermolecular forces between polymer chains.
- The chains can now slide over each other more easily when bent.
For example, polyvinyl chloride (PVC) is the addition polymer formed from chloroethene:
- Unplasticised PVC has long, closely packed chains making it rigid but brittle. It is used in applications such as pipes, window frames, and floor coverings.
- Plasticised PVC has much greater flexibility, allowing uses like cable insulation, shower curtains, and clothing.
The inert nature of addition polymers
Addition polymers are unreactive due to their lack of double bonds and non-polar structure:
- Polyalkenes contain only single covalent bonds. This makes them saturated compounds with maximum stability.
- They also have a carbon backbone lacking any polar groups, giving an overall non-polar molecule.
These factors lead to very low chemical reactivity under normal conditions.