Nuclear Magnetic Resonance (NMR) Spectroscopy
This lesson covers:
- What NMR spectroscopy is and how it provides structural information
- How nuclear spin allows NMR spectroscopy to work
- The concept of chemical environments and chemical shifts
- The use of tetramethylsilane (TMS) as a reference standard
NMR determines molecular structure
Nuclear magnetic resonance (NMR) spectroscopy is a powerful analytical technique that is used to understand the structure of molecules.
There are two main types:
- 13C NMR - This form provides information about the positions of carbon atoms within a molecule.
- 1H NMR - This form provides information about the positions of hydrogen atoms within a molecule.
The basis of NMR involves analysing the changes in magnetic properties of atomic nuclei brought about by their surrounding molecular environments.
Nuclear spin enables NMR
Atomic nuclei that have an odd number of nucleons (protons and/or neutrons) exhibit a characteristic known as nuclear spin.
This nuclear spin transforms the nucleus into a miniature magnet. Notably, hydrogen nuclei (which consist of a single proton) and the carbon isotope 13C (comprising 6 protons and 7 neutrons) both exhibit nuclear spin.
Under normal conditions, the various orientations of nuclear spins negate each other's magnetic fields. However, in the presence of an external magnetic field, these spins tend to align either in the same direction as the field or in the opposite direction.
Nuclei align in two energy levels
Once subjected to an external magnetic field, the nuclei can align in two distinct ways:
- Parallel to the external field, which corresponds to a state of lower energy.
- Anti-parallel to the field, marking a state of higher energy.
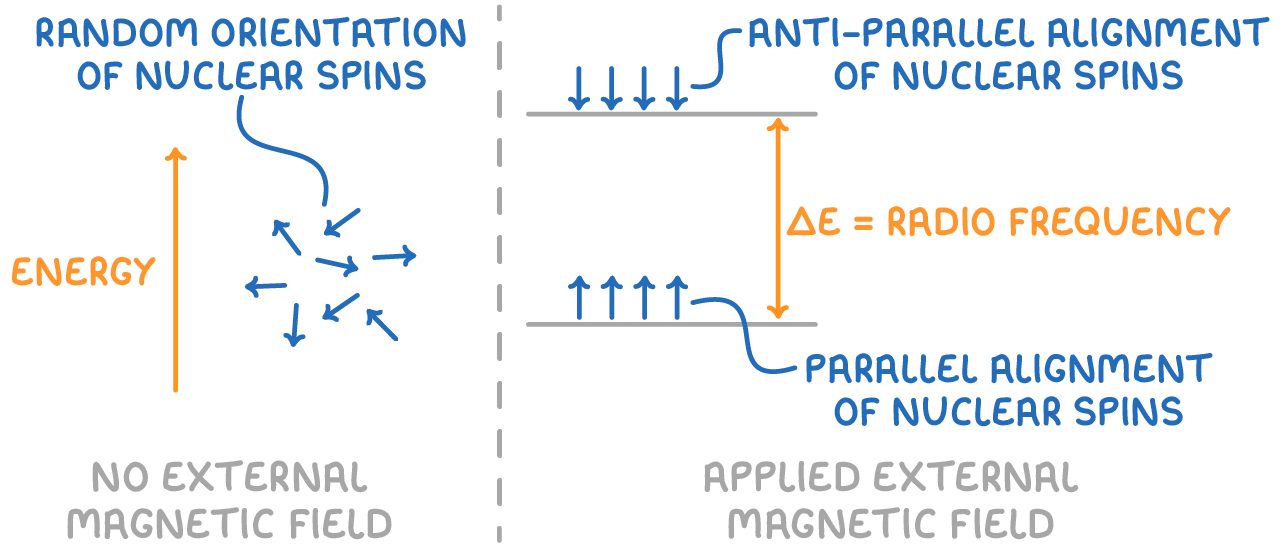
The transition between these two spin states involves the absorption or emission of radiofrequency energy. When the frequency of the applied radiation matches the energy difference between the two spin states, the nuclei resonate and absorb energy, transitioning from the lower to the higher energy level.
NMR spectroscopy measures this process by recording the specific energies absorbed as nuclei resonate and transition between the two energy levels.
Chemical environments influence absorption
The local magnetic shielding experienced by a nucleus, due to the electrons orbiting it within a molecule, protects it from external magnetic fields to a certain extent.
This "chemical environment" encompasses all atoms, functional groups, and bonds that are directly connected to the nucleus within the molecule.
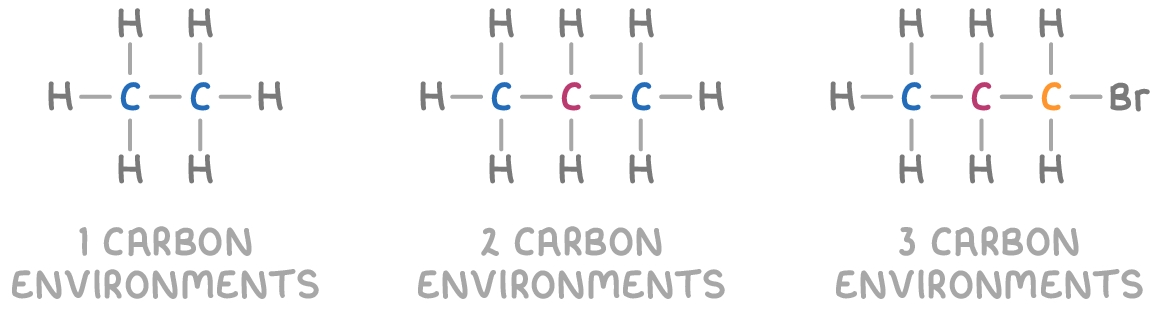
Nuclei situated in different chemical environments will be shielded to varying degrees, leading them to absorb radio waves at slightly different frequencies and energies. The more shielded a nucleus is, the lower its resonance frequency will be, as it requires less energy to flip its spin state in the presence of an external magnetic field.
By analysing these differences in resonance frequencies across various nuclear environments, NMR spectroscopy provides valuable information about the molecular structure.
Chemical shift
In NMR spectroscopy, chemical shift (δ) is a measure of the difference in resonant frequency between a given nucleus and a reference standard. It is expressed in parts per million (ppm) and it increases from right to left on the x-axis of an NMR spectrum.
Tetramethylsilane (Si(CH3)4), or TMS, is used as the standard in NMR spectroscopy because:
- TMS produces a single absorption peak due to all its carbon and hydrogen atoms being in identical chemical environments.
- The TMS peak appears at a lower frequency, located to the right of most analytes on the NMR spectrum, making it an ideal reference point.
The TMS peak is assigned a chemical shift value of 0 ppm, serving as the reference point for measuring chemical shifts of other peaks in the spectrum.
Peaks from the sample that absorb at frequencies higher than that of TMS appear at higher chemical shift values measured relative to the TMS peak at 0 ppm. These peaks are located to the left of the TMS peak, indicating that the corresponding nuclei are less shielded than TMS. Consequently, these nuclei experience a stronger effective magnetic field.