Electrode Potentials and the Electrochemical Series
This lesson covers:
- What electrochemical cells are
- Types of electrochemical cell
- The reversible reactions that occur at each electrode
- Shorthand conventions for representing electrochemical cells
- Measuring electrode potentials against the standard hydrogen electrode
Electrochemical cells convert chemical energy to electrical energy
An electrochemical cell converts chemical energy into electrical energy through redox reactions. It is made up of two half-cells, with each containing an electrode submerged in a solution of ions.
These half-cells are linked by two key components:
- An external circuit - This is a wire that allows the flow of electrons from one electrode to the other, creating an electric current. This current can be measured with a voltmeter.
- A salt bridge - This bridge allows ions to transfer between the half-cells, maintaining charge balance. It's often a filter paper soaked in a neutral electrolyte like potassium nitrate.
The image below shows an electrochemical cell made by connecting a zinc half-cell with a copper-half cell.
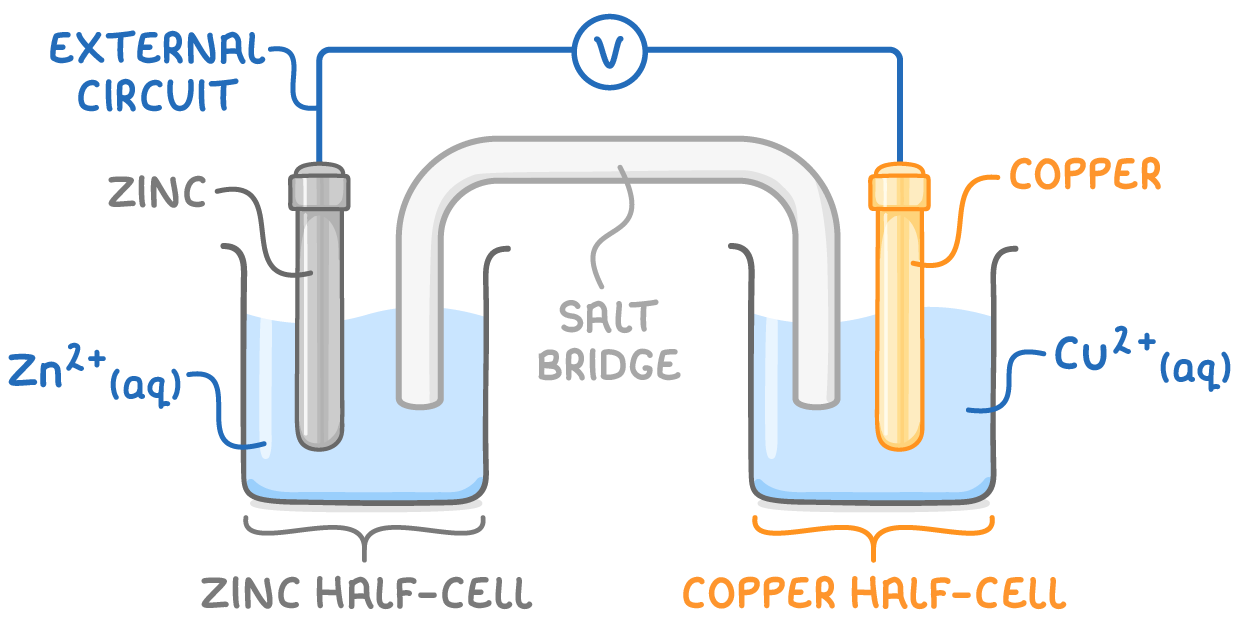
In every electrochemical cell, two half-reactions occur: oxidation happens at the anode (the positive electrode), and reduction happens at the cathode (the negative electrode).
Types of half-cells
Electrochemical cells can be constructed from two main types of half-cells:
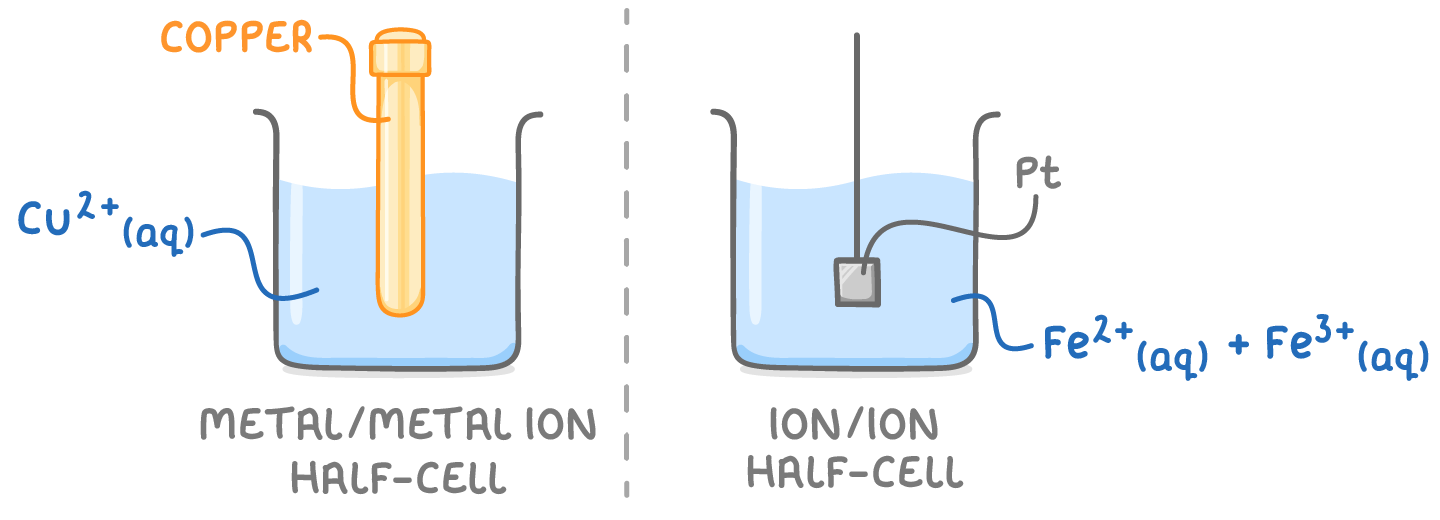
1. Metal/metal ion half-cell:
- Consists of a metal electrode in contact with a solution of its own ions.
- Example: A zinc electrode immersed in a solution of Zn2+ ions.
- The metal can either be oxidised to its ions or the ions can be reduced to the metal, depending on the metal's reactivity.
2. Ion-ion half-cell:
- Consists of two ions of the same element in different oxidation states, in contact with an inert platinum electrode.
- Example: Fe2+ and Fe3+ ions in solution with a platinum electrode.
- The platinum electrode provides an inert surface for the electron transfer between the ions of different oxidation states.
Zinc-copper electrochemical cell
A common example of an electrochemical cell is the zinc-copper cell, which is composed of a zinc half-cell and a copper half-cell:
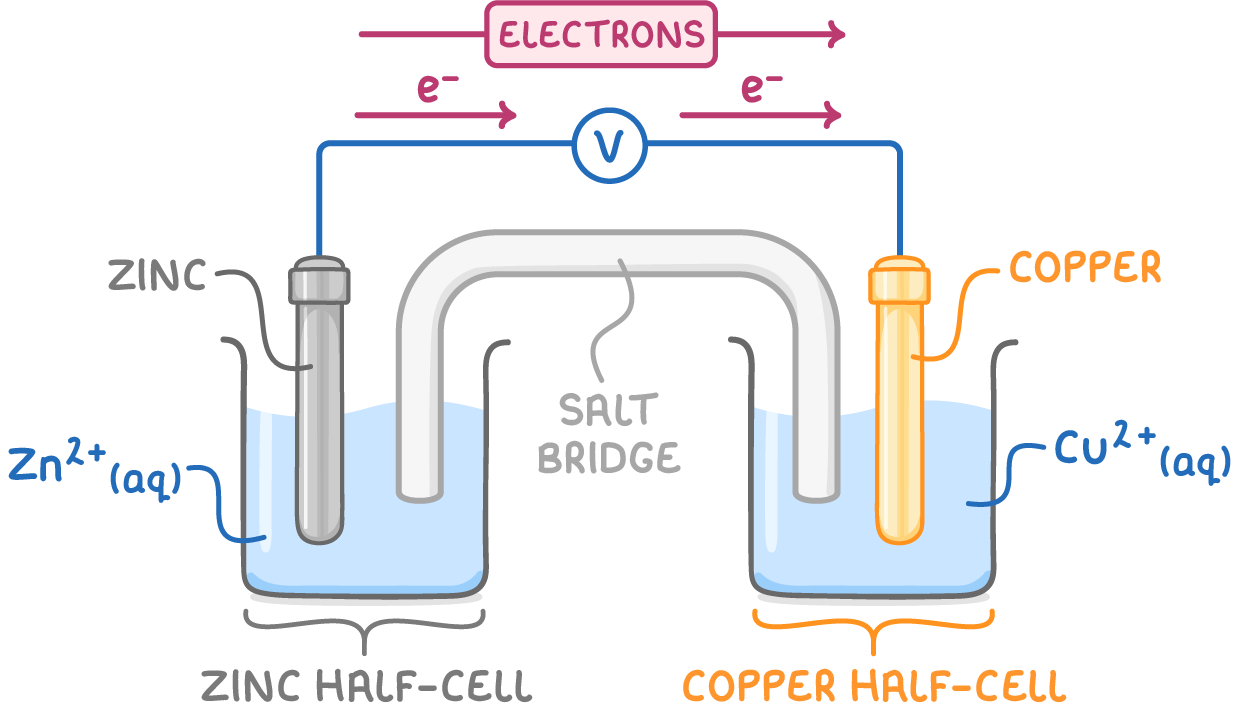
- At the zinc anode, zinc atoms are oxidised to Zn2+ ions, releasing electrons into the external circuit:
Zn(s) ➔ Zn2+(aq) + 2e-
- At the copper cathode, Cu2+ ions are reduced to copper atoms by gaining electrons from the external circuit:
Cu2+(aq) + 2e- ➔ Cu(s)
Electrons move from the zinc, which is more reactive, to the copper, which is less reactive. The voltmeter measures the potential difference between the half-cells, known as the cell potential or electromotive force (e.m.f.), Ecell.
Electrode reactions are reversible
The reaction at each electrode in an electrochemical cell is reversible:
- At the zinc electrode: Zn(s) ⇌ Zn2+(aq) + 2e-
- At the copper electrode: Cu2+(aq) + 2e- ⇌ Cu(s)
The direction of each reaction depends on the ease with which the metal loses electrons (i.e., is oxidised).
This is quantified by the electrode potential of each half-cell:
- Metals that oxidise more readily have more negative electrode potentials.
- Metals that oxidise less readily have less negative or even positive electrode potentials.
| Half-cell | Electrode potential E⦵ (V) |
|---|---|
| Zn2+(aq) + 2e- ⇌ Zn(s) | -0.76 |
| Cu2+(aq) + 2e- ⇌ Cu(s) | +0.34 |
In the zinc-copper cell, since zinc has a more negative electrode potential, it undergoes oxidation (the backward reaction). Copper has a more positive electrode potential, so it undergoes reduction (the forward reaction).
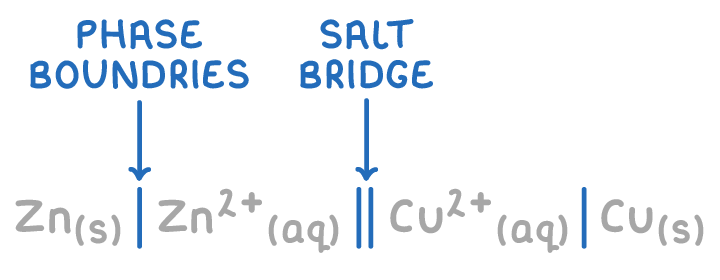
Shorthand notation for electrochemical cells
Electrochemical cells can be represented using a concise shorthand notation.
For the zinc-copper cell:
Zn(s) | Zn2+(aq) || Cu2+(aq) | Cu(s)
Key features of this notation include:
- The half-cell with the more negative potential (i.e. Zn/Zn2+) is listed on the left.
- A single vertical line indicates a phase boundary between the metal electrode and the aqueous ion.
- A double vertical line denotes the salt bridge.
- The oxidised species (i.e. Zn2+ and Cu2+) are written next to the salt bridge.
By following this convention, the cell potential (E⦵cell) is calculated from the standard electrode potentials (E⦵) of the two half-cells:
Ecell ⊖=Eright−hand side ⊖−Eleft−hand side ⊖
For the zinc-copper cell:
E⦵cell = +0.34−(−0.76)=+1.10 V
Standard hydrogen electrode as reference
The standard hydrogen electrode acts as a universal reference for measuring electrode potentials. It features a platinum electrode immersed in a 1.00 mol dm-3 solution of H+(aq) ions at 298 K, with H2 gas at a pressure of 100 kPa bubbling over the electrode.
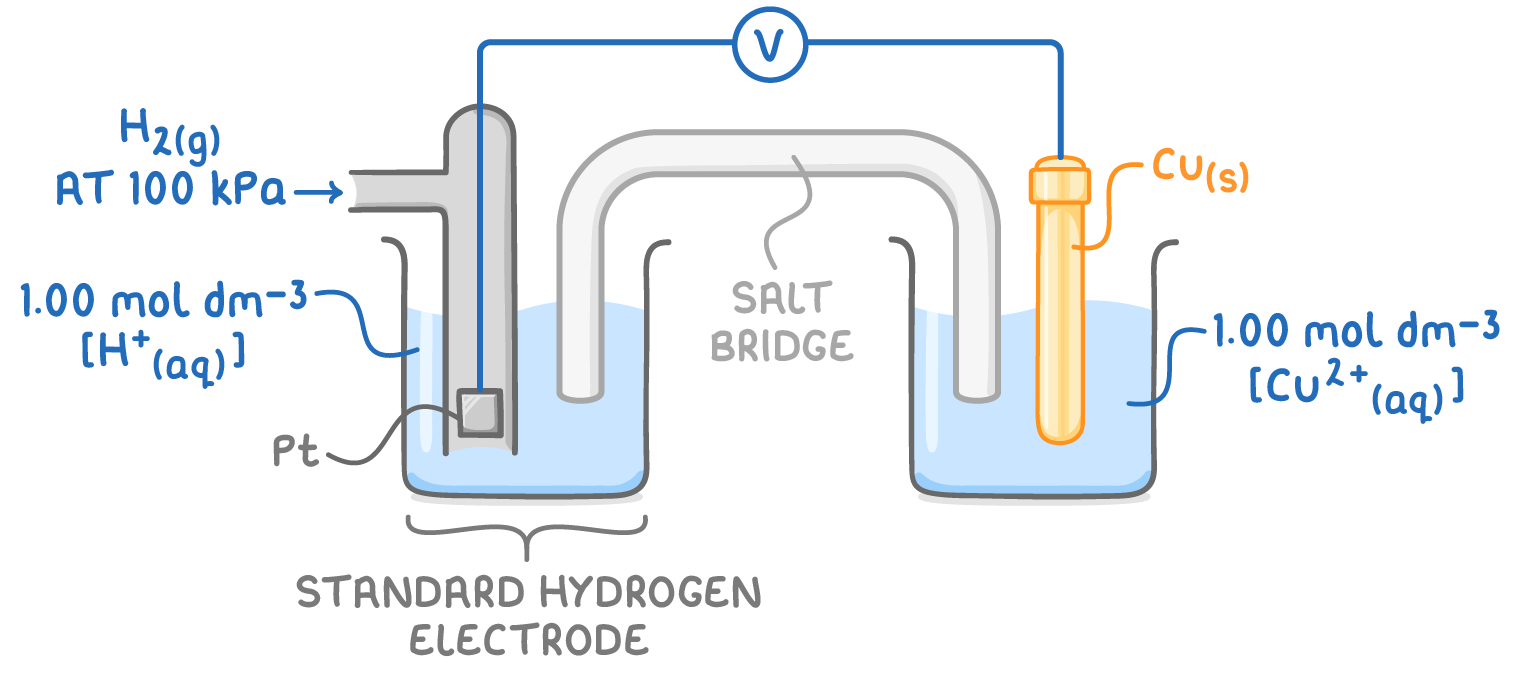
The standard hydrogen electrode has a standard electrode potential (E⦵) set at 0.00 V.
To determine the E⦵ value of another half-cell, it's connected to the standard hydrogen electrode under standard conditions:
- The temperature is kept at 298 K (25°C).
- The pressure is kept at 100 kPa.
- Solutions are at a concentration of 1.00 mol dm-3 or are equimolar.
The ⦵ symbol beside E indicates these standard conditions are met.
In diagrams, the standard hydrogen electrode is always positioned on the left side, regardless of the relative E⦵ values of the half-cells. The voltmeter's reading reflects the E⦵ value of the other half-cell, which can be positive or negative, depending on the electron flow direction relative to the standard hydrogen electrode.
The standard electrode potential (E⦵) of a half-cell is the voltage measured under standard conditions when the half-cell is connected to a standard hydrogen electrode
Ecell ⊖=Eright−hand side ⊖−Eleft−hand side ⊖
Ecell ⊖=Eright−hand side ⊖−0.00
Ecell ⊖=Eright−hand side⊖
If conditions are not standard, the measured cell potential may vary from the E⦵cell value. Nonetheless, the standard hydrogen electrode remains a consistent reference, allowing for the comparison of electrode potentials across different half-cells.