The Oxides of Elements in Period 3
This lesson covers:
- The bonding and structures of oxides
- How bonding and structure affect oxide melting points
- How oxides of period 3 elements behave in reactions with water
Bonding and structure of period 3 oxides
The oxides of period 3 elements show a range of different types of bonding and structures:
- Na2O and MgO are ionic metal oxides that form giant ionic lattice structures. This is because the large electronegativity difference between the metal and oxygen atoms creates strong strong ionic bonds between oppositely charged ions.
- Al2O3 is an ionic oxide that forms a giant ionic lattice structure with mixed bonding. The small, highly charged Al3+ ion can get close to the O2- ion and distort its electron cloud, resulting in the formation of a partial covalent bond between Al and O.
- SiO2 forms a giant covalent network with a continuous, strong covalent structure extending in all directions. Each silicon atom shares electrons with four oxygen atoms and each oxygen shares electrons with two silicon atoms.
- P4O10 and SO2 form simple molecular structures where discrete molecules are held together by weak intermolecular forces. Within each molecule there are strong covalent P-O and S-O bonds.
Melting points relate to bonding and structure
The strength of bonds in an oxide determines the amount of energy needed to melt it from a solid to a liquid. The stronger the bonds in an oxide, the more energy is required to break those bonds and the higher its melting point.
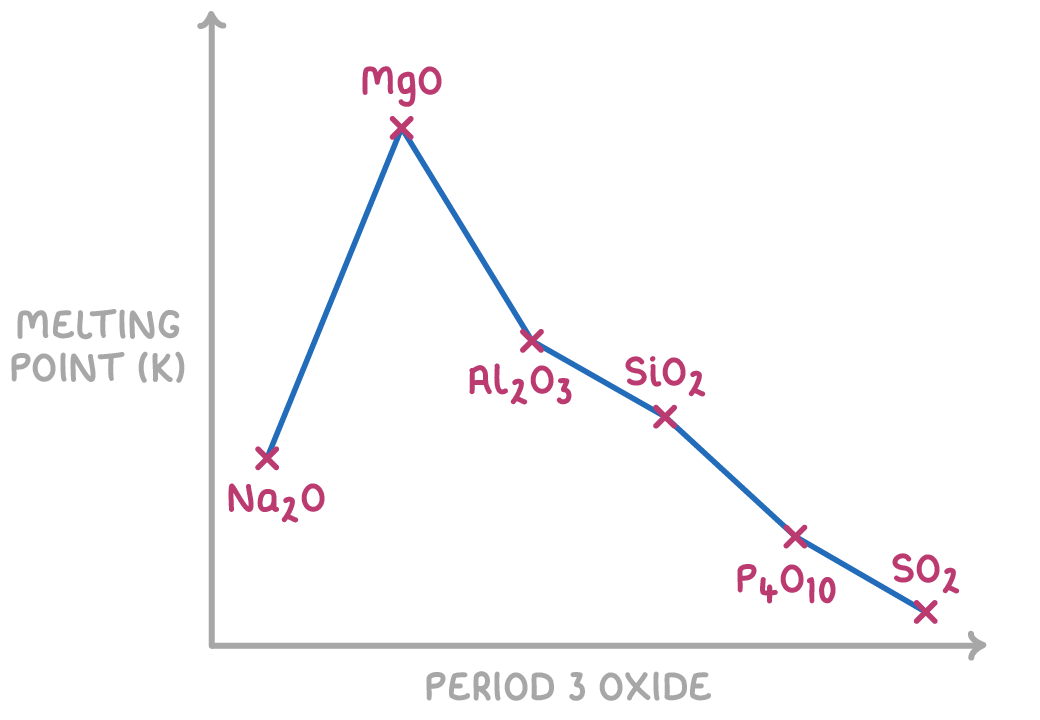
The ionic oxides Na2O, MgO and Al2O3 have the highest melting points as their lattice structures are held together by the strongest electrostatic forces between oppositely charged ions which require the most amount of energy to overcome.
- MgO has a higher melting point than Na2O because the Mg2+ ion is smaller and more highly charged than the Na+ ion. This creates stronger electrostatic attractions between ions in the MgO lattice structure.
- Al2O3 has a lower melting point than MgO because the ionic bonding in Al2O3 has some covalent character, meaning the electrostatic forces between ions are weaker.
SiO2 has the next highest melting point because of the many strong covalent silicon-oxygen bonds in its giant covalent structure.
The simple molecular oxides P4O10 and SO2 have the lowest melting points as their molecules are held together by weak intermolecular forces which require the least amount of energy to overcome.
- P4O10 has a slightly higher melting point than SO2 because it forms larger molecules with more electrons available to create stronger induced dipole-dipole forces between P4O10 molecules.
Reactions of period 3 oxides with water
The oxides show different reactions with water due to differences in their structure and bonding.
The table below summarises the bonding, structure and reactions with water for various period 3 oxides:
| Oxide | Na2O | MgO | Al2O3 | SiO2 | P4O10 | SO2 | SO3 |
|---|---|---|---|---|---|---|---|
| Bonding type | Ionic | Ionic | Ionic (with degree of covalent character) | Covalent | Covalent | Covalent | Covalent |
| Reaction with water | Dissolves | Dissolves | Insoluble | Insoluble | Reacts violently | Reacts violently | Reacts violently |
| pH of solution | 12-14 | 10 | 7 | 7 | 1-2 | 2-3 | 0-1 |
The general trend is that the solutions of the oxides of period 3 go from alkaline to acidic as you move across period 3.
Sodium and magnesium oxides form alkaline solutions
Na2O and MgO have giant ionic lattice structures. When dissolved in water, these lattices separate into hydrated ions:
- Na2O(s) ➔ 2Na+(aq) + O2−(aq)
- MgO(s) ➔ Mg2+(aq) + O2−(aq)
The oxide ions (O2−) react with water molecules to form hydroxide ions (OH−):
- O2−(aq) + H2O(l) ➔ 2OH−(aq)
This makes the solution alkaline. For example:
- Na2O(s) + H2O(l) ➔ 2NaOH(aq) (pH 12-14)
- MgO(s) + H2O(l) ➔ Mg(OH)2(aq) (pH ≈10)
Aluminium oxide and silicon dioxide are insoluble
- Al2O3 has an ionic structure with some covalent character. It does not dissolve or react with water partly due to the additional covalent bonding it has.
- SiO2 has a giant covalent structure, so it does not dissolve or react with water.
Phosphorus pentoxide and sulfur oxides form acidic solutions
P4O10, SO2 and SO3 have simple molecular structures.
When water is added, they react vigorously and dissolve to form acidic solutions containing hydrogen ions (H+) that are donated as the acidic oxides dissolve.
- Phosphorus pentoxide P4O10 dissolves in water to form phosphoric(V) acid: P4O10(s) + 6H2O(l) ➔ 4H3PO4(aq)
This reaction produces an acidic solution with a pH of 2.
- Sulfur dioxide SO2 dissolves in water to form sulfurous acid: SO2(g) + H2O(l) ➔ H2SO3(aq)
- Sulfur trioxide SO3 dissolves in water to form sulfuric acid: SO3(g) + H2O(l) ➔ H2SO4(aq)
- The sulfur oxides reacting with water create acidic solutions with a pH of 0-3.