Elimination Reactions in Halogenoalkanes
This lesson covers:
- How halogenoalkanes undergo elimination reactions
- The elimination reaction mechanism
- How changing conditions influences if substitution or elimination occurs
Halogenoalkanes eliminate under alkaline conditions
If a halogenoalkane is heated under reflux with an alkali like potassium hydroxide (KOH) dissolved in ethanol, an elimination reaction occurs to form an alkene.
For example, heating 2-iodopropane with KOH gives propene:
CH3CHICH3 + KOH ➔ CH2=CHCH3 + H2O + KI
This is an example of an elimination reaction, where a small group breaks off a larger molecule and is not replaced.
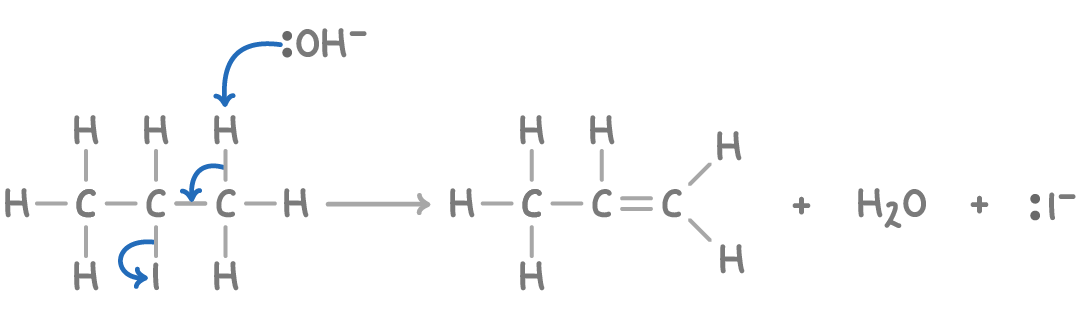
Reaction mechanism
The mechanism of the elimination reaction occurs in three steps:
- OH- removes a hydrogen ion (H+) from the halogenoalkane, forming water.
- This leaves the adjacent carbon with a spare electron to form a π bond.
- The movement of electrons breaks the carbon-halogen bond heterolytically, eliminating the halide ion.
So OH- acts as a base in elimination reactions by removing a proton.
The mechanism for the elimination of 2-iodopropane with OH- is:
Anhydrous conditions favour elimination
Halogenoalkanes treated with hydroxide can undergo either substitution or elimination, depending on the choice of solvent.
- Substitution is favoured in aqueous conditions. OH- acts as a nucleophile.
- Elimination is favoured in ethanolic conditions. OH- acts as a base.
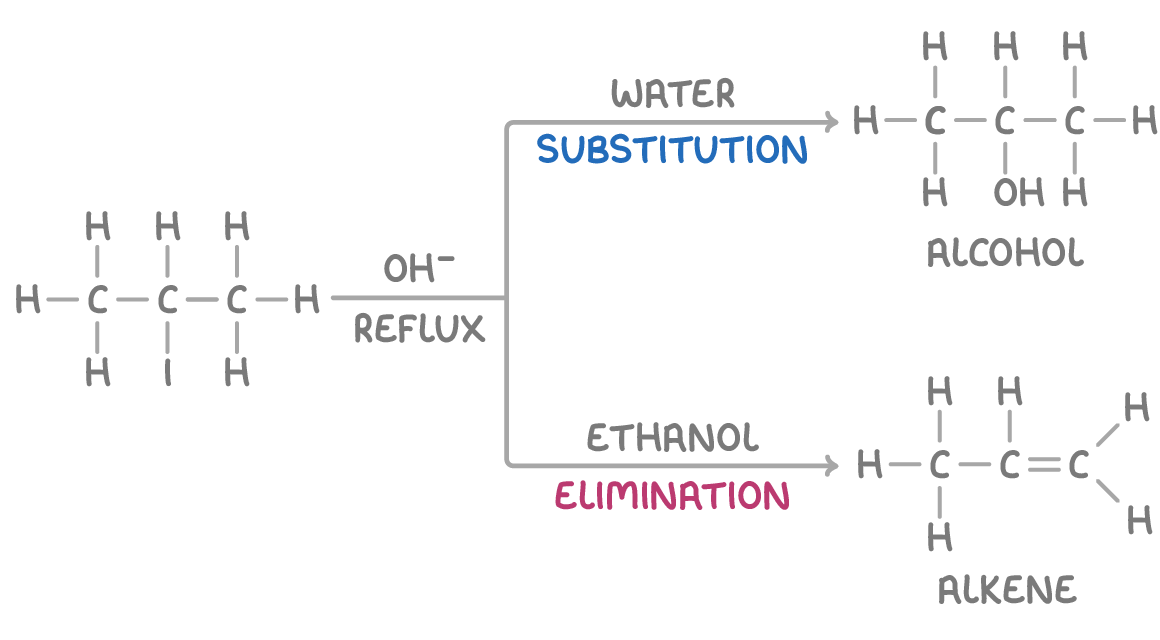
Using a solvent mixture of water and alcohol allows both reactions to occur, giving a mixture of products.