Hess' Law
This lesson covers:
- What Hess' law states
- How reaction enthalpies can be calculated using enthalpies of formation
- How reaction enthalpies can be calculated using enthalpies of combustion
Hess' law says route doesn't affect ΔH
Hess' law states that the overall standard enthalpy change (ΔH⦵) of a reaction is the same, regardless of whether the reaction takes place in one step or several steps.
This allows you to break a reaction into component steps and add up their enthalpy changes to find the total ΔH⦵ for the overall reaction.
Using enthalpies of formation
Standard enthalpies of formation (ΔH⦵f) can be used with Hess' law to determine unknown ΔH⦵ values.
- ΔH⦵f values are known for many compounds
- ΔH⦵f for elements in their standard states is defined as 0 kJ mol−1
To calculate the standard enthalpy change of a reaction (ΔH⦵r), use the equation:
ΔH⦵r = ΣΔH⦵f(products) - ΣΔH⦵f(reactants)
where Σ = sum of
Worked example 1 - Calculating ΔH⦵ using enthalpy of formation data
Calculate ΔH⦵r for the reaction:
SO2(g) + 2H2S(g) ➔ 3S(s) + 2H2O(l)
Given:
ΔH⦵f(SO2(g) = -297 kJ mol-1
ΔH⦵f(H2S(g)) = -20.2 kJ mol-1
ΔH⦵f(H2O(l)) = -286 kJ mol-1
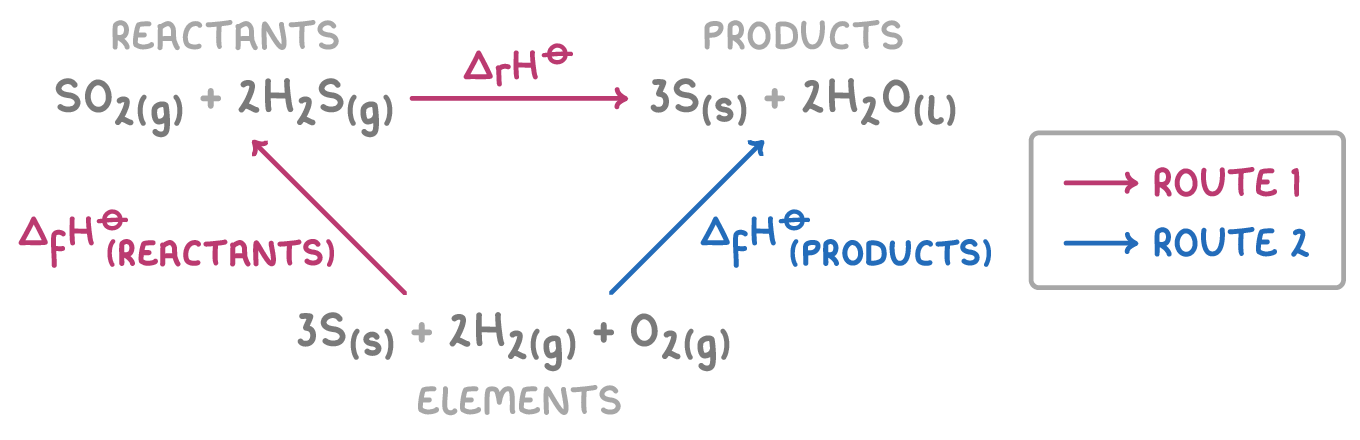
This diagram shows the reaction pathway from reactants to products. We can use Hess' law and known enthalpy values to calculate ΔH⦵ for this reaction.
Step 1: Equation
ΔH⦵r = ΣΔH⦵f(products) - ΣΔH⦵f(reactants)
Step 2: Substitution and correct evaluation
ΔH⦵f(products) = 3(0) + 2(-286) = -572 kJ mol-1
ΔH⦵f(reactants) = -297 + 2(-20.2) = -337.4 kJ mol-1
Step 3: Determine the overall ΔH⦵
ΔH⦵ = -572 + 337.4 = -234.6 kJ mol-1
Therefore, the ΔH⦵ for the reaction is -235 kJ mol-1
Using enthalpies of combustion
In a similar way, standard enthalpies of combustion can be used to find unknown ΔH⦵r values for reactions.
To calculate the standard enthalpy change of a reaction (ΔH⦵r), use the equation:
ΔH⦵r = ΣΔH⦵c(reactants) - ΣΔH⦵c(products)
where Σ = sum of
Worked example 2 - Calculating ΔH⦵ using enthalpy of combustion data
Calculate ΔH⦵r for the reaction:
C2H5OH(l) + 3O2(g) ➔ 2CO2(g) + 3H2O(l)
Given:
ΔH⦵c(C2H5OH) = -1,367 kJ mol−1
ΔH⦵c(C) = -394 kJ mol−1
ΔH⦵c(H2) = -286 kJ mol−1
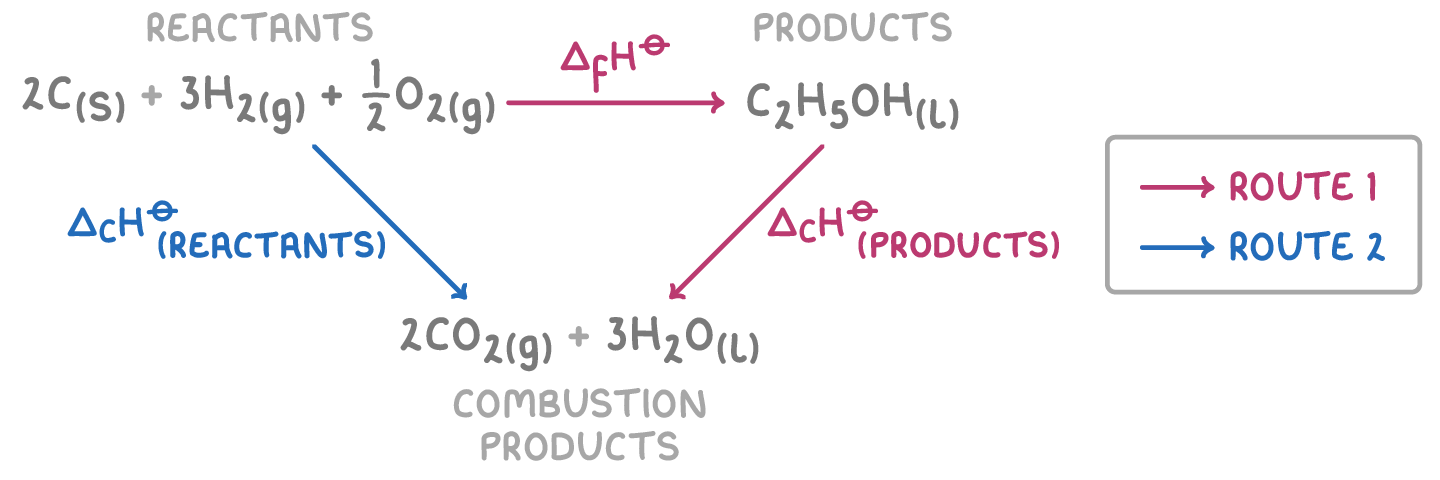
This diagram shows the reaction pathway from reactants to products. We can use Hess' law and known enthalpy values to calculate ΔH⦵ for this reaction.
Step 1: Equation
ΔH⦵r = ΣΔH⦵c(reactants) - ΣΔH⦵c(products)
Step 2: Substitution and correct evaluation
ΔH⦵c(reactants) = 2(-394) + 3(-286) = -1,646 kJ mol-1
ΔH⦵c(products) = -1,367 kJ mol-1
Step 3: Determine the overall ΔH⦵
ΔH⦵ = -1,646 - (-1,367) = -279 kJ mol-1
Therefore, the ΔH⦵ for the formation of ethanol is -279 kJ mol-1.