Isomerism
This lesson covers:
- Structural isomers and stereoisomers
- The three types of structural isomer: chain, positional, and functional group
- The two types of stereoisomer: geometric and optical
- How to identify structural isomers and stereoisomers
- E/Z system for naming stereoisomers
- Using Cahn-Ingold-Prelog priority rules to identify E/Z isomers
Types of isomerism
Isomers are molecules with the same molecular formula but different arrangements of atoms.
They fall into two main categories:
- Structural isomers - These molecules have the same atoms but different connections.
- Stereoisomers - These molecules are connected in the same way but have different spatial arrangements of atoms.
Structural isomers
Structural isomers are divided into three sub-types:
- Chain isomers - Differ in the carbon skeleton arrangement (e.g., straight chain vs branched chain).
- Positional isomers - The functional group is attached at different carbon atoms.
- Functional group isomers - The atoms form different functional groups.
Worked example 1 - Identifying chain isomers in butane
Determine the possible chain isomers of butane.
Step 1: Identify the molecular formula
Butane has the molecular formula C4H10.
Step 2: Determine possible structures
- Butane - A straight chain of four carbon atoms.
- Methylpropane - A branched chain with three carbon atoms in the main chain and one methyl group branching off.
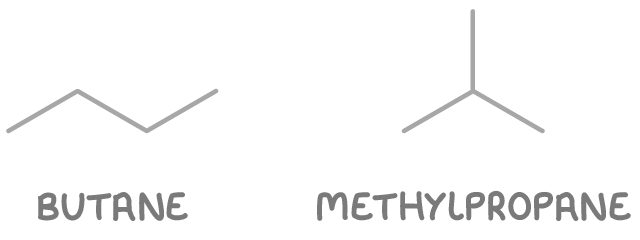
So there are two chain isomers of C4H10: butane and methylpropane.
Worked example 2 - Identifying positional isomers of chloropentane
Determine the positional isomers of chloropentane.
Step 1: Identify the molecular formula
Chloropentane has the molecular formula C5H11Cl.
Step 2: Determine possible structures
- 1-chloropentane - Chlorine on the first carbon.
- 2-chloropentane - Chlorine on the second carbon.
- 3-chloropentane - Chlorine on the third carbon.
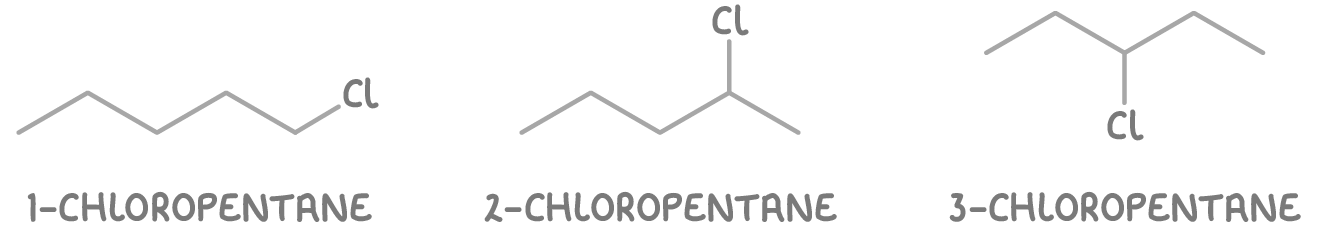
So there are three positional isomers of chloropentane: 1-chloropentane, 2-chloropentane, and 3-chloropentane.
Note: 4-chloropentane and 5-chloropentane mirror 2-chloropentane and 1-chloropentane due to the symmetry of the pentane chain, so they aren't distinct isomers.
Worked example 3 - Identifying functional group isomers of C3H6O
Determine the functional group isomers for C3H6O.
Step 1: Identify functional groups and carbon skeleton
C3H6O can form different functional groups like aldehydes or ketones.
Step 2: Determine possible structures
- Propanal - An aldehyde at the end of a three-carbon chain.
- Propanone - A ketone in the middle of a three-carbon chain.
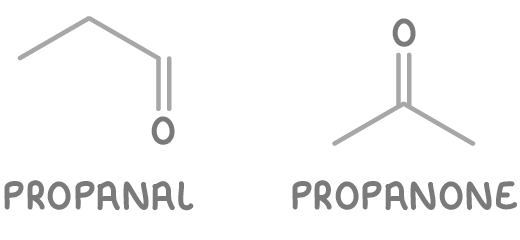
So C3H6O has two functional group isomers: propanal, and propanone.
Stereoisomers
Stereoisomers are categorised into two sub-types:
- Geometric (E/Z) isomers
- Optical isomers
Geometric (E/Z) isomers
Geometric isomers, also known as E/Z isomers, occur due to restricted rotation around carbon-carbon double bonds in alkenes. There are two possible arrangements:
- Z-isomer - Higher priority groups are on the same side (above or below) of the double bond.
- E-isomer - Higher priority groups are across the double bond from each other.
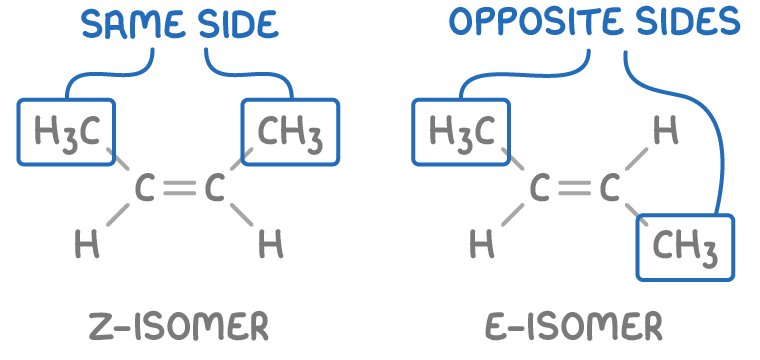
For example, in Z-but-2-ene, the higher priority methyl groups are positioned above the same side of the double bond, whereas in E-but-2-ene, the methyl groups are positioned diagonally across the double bond from one another.
Identifying E/Z isomers
- Use Cahn-Ingold-Prelog priority rules to assign priority to groups bonded to each carbon of the double bond. Higher atomic number means higher priority.
- Check if the highest priority groups are across (E) or on the same side (Z) of the double bond.
Worked example 4 - Identifying E/Z stereoisomers
Classify the alkene below using E/Z terminology.
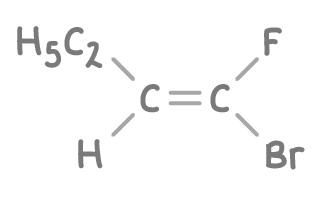
Step 1: Examine the molecule
The alkene has a double bond between two carbon atoms. One carbon is attached to an ethyl group and a hydrogen atom, the other to a bromine atom and a fluorine atom.
Step 2: Apply Cahn-Ingold-Prelog priority rules
- Compare directly attached atoms to the double-bonded carbons.
- Higher atomic number means higher priority.
Step 3: Determine priority groups on each carbon
- First carbon: Ethyl group (C) has a higher atomic number than Hydrogen (H).
- Second carbon: Bromine (Br) has a higher atomic number than Fluorine (F).
Step 4: Analyse arrangement of high priority groups
The high priority groups (ethyl and bromine) are positioned on opposite sides of the double bond.
Therefore, the molecule is the E-isomer.
Optical isomers
Optical isomers form when a molecule contains a chiral centre - a carbon atom bonded to four different substituents, creating non-superimposable mirror image forms, or enantiomers.
For instance, 2-chlorobutane has a chiral centre on its second carbon, bonded to a chlorine atom, a methyl group, an ethyl group, and a hydrogen atom, resulting in two mirror image forms.
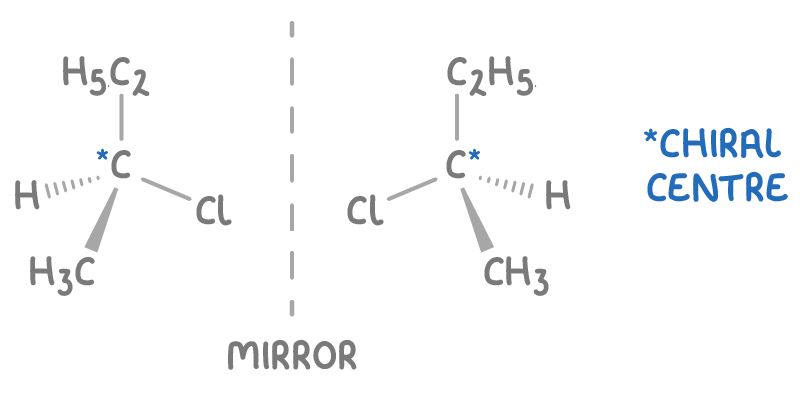
These enantiomers, though structurally identical, show different interactions with plane-polarised light.