The Nature of Ionic Bonding
This lesson covers:
- How ions form through electron transfer
- Compound ions
- How to determine the formula of ionic compounds
- How ionic bonds are formed
- The structure and properties of ionic compounds
Ions form to achieve full outer electron shells
Atoms can gain or lose electrons to form ions with full outer shells:
Metal atoms lose electrons to become positive ions called cations. For example, sodium loses one electron to form a sodium ion (Na+).
- Sodium atom: 1s2, 2s2, 2p6, 3s1
- Sodium ion (Na+): 1s2, 2s2, 2p6
Non-metal atoms gain electrons to become negative ions called anions. For example, chlorine gains one electron to form a chloride ion (Cl-).
- Chlorine atom: 1s2, 2s2, 2p6, 3s2, 3p5,
- Chloride ion (Cl-): 1s2, 2s2, 2p6, 3s2, 3p6,
In ionic bonding, electrons lost by the metal atom are transferred to the non-metal atom. This transfer allows both atoms to achieve full outer electron shells, resulting in the formation of stable ions.
Dot and cross diagrams show how electrons are transferred to form ions:
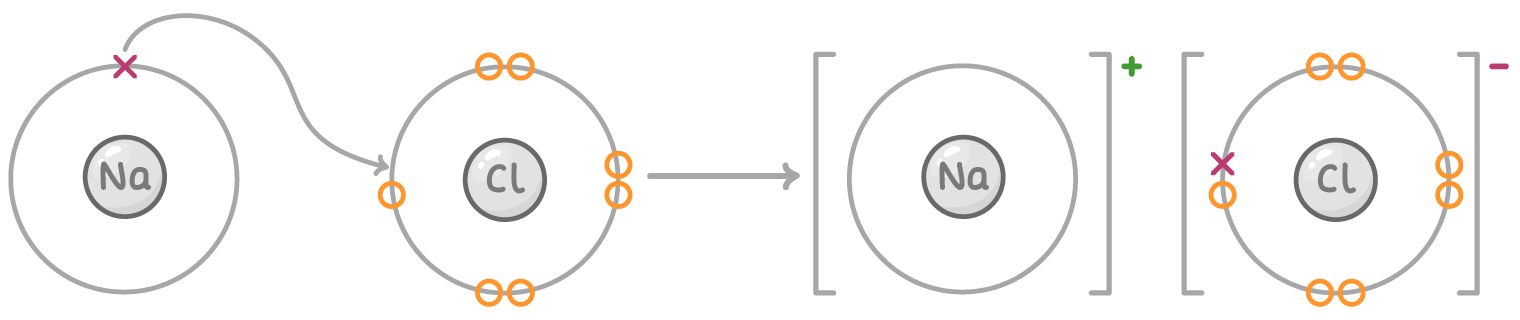
Elements in the same group of the periodic table form ions with the same charge as they have the same number of outer shell electrons:
- Elements in group 1 form 1+ ions
- Elements in group 2 form 2+ ions
- Elements in group 6 form 2- ions
- Elements in group 7 form 1- ions
Unlike main group elements, transition elements have partially filled d sub-shells, enabling them to form multiple ions with different charges. For example, iron can form both Fe2+ and Fe3+ ions:
- Fe2+: [Ar] 3d6
- Fe3+: [Ar] 3d5
When transition elements undergo ionisation, the 4s electrons are lost before the 3d electrons. The similar energies of the 3d and 4s sub-shells allow for successive ionisations in many transition elements, resulting in the formation of ions with varying charges.
Compound ions
Compound ions consist of atoms from two or more elements chemically bonded together, resulting in an overall charge.
The important compound ions to know are:
- Nitrate ion (NO3-)
- Carbonate ion (CO32-)
- Sulfate ion (SO42-)
- Hydroxide ion (OH-)
- Ammonium ion (NH4+)
Determining formulae of ionic compounds
Ionic compound formulas indicate the ions present and their ratios. For example:
- NaCl contains Na+ ions and Cl- ions in a 1:1 ratio
- MgCl2 contains Mg2+ ions and Cl- ions in a 1:2 ratio
The total positive and negative charges must balance in an ionic compound.
To determine the formula of an ionic compound from its name:
- Identify the cation and anion based on the name.
- Determine the charges of the cation and anion.
- Balance the charges to make the compound electrically neutral by adjusting the ratios of the ions.
Worked example 1 - Determining the formula of an ionic compound
Deduce the formula of aluminium oxide.
Step 1: Identify ions present
- Aluminium ion
- Oxide ion
Step 2: Determine charges of each ion
- Al3+
- O2-
Step 3: Balance positive and negative charges
The smallest number 3+ and 2- charges can balance at is 6, so:
- Two aluminium ions (2 x Al3+) have a total charge of +6
- Three oxide ions (3 x O2-) have a total charge of -6
Therefore, the formula of aluminium oxide is Al2O3.
Ionic bonding occurs between oppositely charged ions
- An ionic bond is the electrostatic force of attraction between oppositely charged ions, usually a metal and a non-metal.
- These bonds are very strong.
- When ions bond this way, an ionic compound is formed.
Structure of ionic compounds
Ionic compounds have giant lattice structures where positive and negative ions pack together.
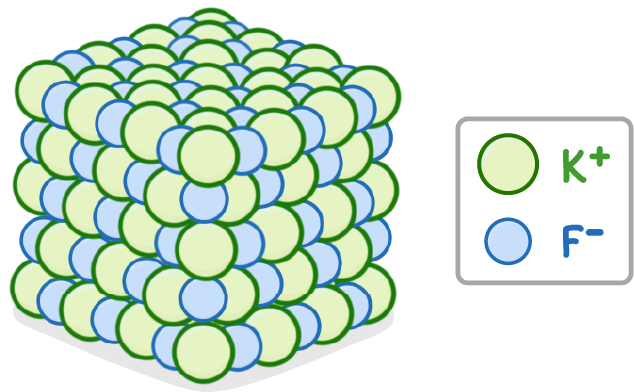
A 3D model of potassium fluoride’s giant ionic lattice structure with alternating K+ and F- ions is shown above.
Key features of a giant ionic lattice:
- Each ion is electrostatically attracted to ions of the opposite charge in all directions.
- It takes significant energy to overcome these strong electrostatic forces between the ions.
Properties related to ionic structure
The properties of ionic compounds result from their lattice structure:
- High melting and boiling points - The strong electrostatic attractions between the positive and negative ions in the giant lattice must be overcome for the lattice to break apart; this requires a lot of energy.
- Conduct electricity when molten or in solution - When melted or dissolved, the ions can move freely and carry electric charge through the liquid.
- Do not conduct electricity as solids - In the solid lattice structure, the ions are firmly locked in place and unable to move to carry electric charge.
- Dissolve in water - Water molecules, which are polar, attract the charged ions in the lattice via ion-dipole forces, pulling them away from the lattice and dissolving the structure.